Biomedical and Catalytic Opportunities of Virus-Like Particles in Nanotechnology
- PMID: 28057256
- PMCID: PMC5384350
- DOI: 10.1016/bs.aivir.2016.09.002
Biomedical and Catalytic Opportunities of Virus-Like Particles in Nanotechnology
Abstract
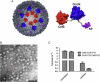
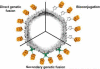
Within biology, molecules are arranged in hierarchical structures that coordinate and control the many processes that allow for complex organisms to exist. Proteins and other functional macromolecules are often studied outside their natural nanostructural context because it remains difficult to create controlled arrangements of proteins at this size scale. Viruses are elegantly simple nanosystems that exist at the interface of living organisms and nonliving biological machines. Studied and viewed primarily as pathogens to be combatted, viruses have emerged as models of structural efficiency at the nanoscale and have spurred the development of biomimetic nanoparticle systems. Virus-like particles (VLPs) are noninfectious protein cages derived from viruses or other cage-forming systems. VLPs provide incredibly regular scaffolds for building at the nanoscale. Composed of self-assembling protein subunits, VLPs provide both a model for studying materials' assembly at the nanoscale and useful building blocks for materials design. The robustness and degree of understanding of many VLP structures allow for the ready use of these systems as versatile nanoparticle platforms for the conjugation of active molecules or as scaffolds for the structural organization of chemical processes. Lastly the prevalence of viruses in all domains of life has led to unique activities of VLPs in biological systems most notably the immune system. Here we discuss recent efforts to apply VLPs in a wide variety of applications with the aim of highlighting how the common structural elements of VLPs have led to their emergence as paradigms for the understanding and design of biological nanomaterials.
Keywords: Bioconjugation; Biomaterials; Biomimicry; Confined catalysis; Confined polymerization; Nanomaterials; Vaccine; Virus-like particle.
© 2017 Elsevier Inc. All rights reserved.
Figures









References
-
- Aime S, Frullano L, Geninatti Crich S. Compartmentalization of a gadolinium complex in the apoferritin cavity: a route to obtain high relaxivity contrast agents for magnetic resonance imaging. Angew. Chem. Int. Ed. 2002;41:1017–1019. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Allen M, Willits D, Young M, Douglas T. Constrained synthesis of cobalt oxide nanomaterials in the 12-subunit protein cage from Listeria innocua. Inorg. Chem. 2003;42:6300–6305. - PubMed
-
- Allen M, et al. Paramagnetic viral nanoparticles as potential high-relaxivity magnetic resonance contrast agents. Magn. Reson. Med. 2005;54:807–812. - PubMed
-
- Anderson EA, et al. Viral nanoparticles donning a paramagnetic coat: conjugation of MRI contrast agents to the MS2 capsid. Nano Lett. 2006;6:1160–1164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

