Nanomedicines for dysfunctional macrophage-associated diseases
- PMID: 28057522
- PMCID: PMC5360184
- DOI: 10.1016/j.jconrel.2016.12.032
Nanomedicines for dysfunctional macrophage-associated diseases
Abstract
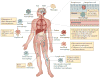
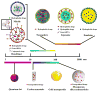
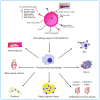
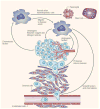
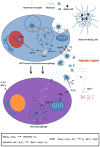
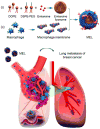
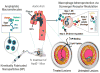
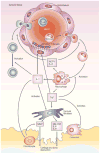
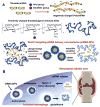
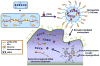
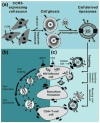
Macrophages play vital functions in host inflammatory reaction, tissue repair, homeostasis and immunity. Dysfunctional macrophages have significant pathophysiological impacts on diseases such as cancer, inflammatory diseases (rheumatoid arthritis and inflammatory bowel disease), metabolic diseases (atherosclerosis, diabetes and obesity) and major infections like human immunodeficiency virus infection. In view of this common etiology in these diseases, targeting the recruitment, activation and regulation of dysfunctional macrophages represents a promising therapeutic strategy. With the advancement of nanotechnology, development of nanomedicines to efficiently target dysfunctional macrophages can strengthen the effectiveness of therapeutics and improve clinical outcomes. This review discusses the specific roles of dysfunctional macrophages in various diseases and summarizes the latest advances in nanomedicine-based therapeutics and theranostics for treating diseases associated with dysfunctional macrophages.
Keywords: Cell receptor; Drug delivery; Gene therapy; In vivo; Macrophage phenotype; Tissue macrophages.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures



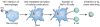
Similar articles
-
Applying nanomedicine in maladaptive inflammation and angiogenesis.Adv Drug Deliv Rev. 2017 Sep 15;119:143-158. doi: 10.1016/j.addr.2017.05.009. Epub 2017 May 12. Adv Drug Deliv Rev. 2017. PMID: 28506745 Free PMC article. Review.
-
Nanomedicine delivers promising treatments for rheumatoid arthritis.Nanomedicine (Lond). 2015;10(13):2063-74. doi: 10.2217/nnm.15.45. Epub 2015 Jun 18. Nanomedicine (Lond). 2015. PMID: 26084368 Free PMC article. Review.
-
Macrophage targeted theranostics as personalized nanomedicine strategies for inflammatory diseases.Theranostics. 2015 Jan 1;5(2):150-72. doi: 10.7150/thno.9476. eCollection 2015. Theranostics. 2015. PMID: 25553105 Free PMC article. Review.
-
Efficient Targeting of Adipose Tissue Macrophages in Obesity with Polysaccharide Nanocarriers.ACS Nano. 2016 Jul 26;10(7):6952-62. doi: 10.1021/acsnano.6b02878. Epub 2016 Jun 23. ACS Nano. 2016. PMID: 27281538
-
Recent advances in nanomedicines for the treatment of rheumatoid arthritis.Biomater Sci. 2017 Jul 25;5(8):1407-1420. doi: 10.1039/c7bm00254h. Biomater Sci. 2017. PMID: 28631779 Review.
Cited by
-
Recent progress of macrophage vesicle-based drug delivery systems.Drug Deliv Transl Res. 2022 Oct;12(10):2287-2302. doi: 10.1007/s13346-021-01110-5. Epub 2022 Jan 4. Drug Deliv Transl Res. 2022. PMID: 34984664 Review.
-
Nanoparticle targeting of de novo profibrotic macrophages mitigates lung fibrosis.Proc Natl Acad Sci U S A. 2022 Apr 12;119(15):e2121098119. doi: 10.1073/pnas.2121098119. Epub 2022 Apr 4. Proc Natl Acad Sci U S A. 2022. PMID: 35377803 Free PMC article.
-
Targeting of immunosuppressive myeloid cells from glioblastoma patients by modulation of size and surface charge of lipid nanocapsules.J Nanobiotechnology. 2020 Feb 17;18(1):31. doi: 10.1186/s12951-020-00589-3. J Nanobiotechnology. 2020. PMID: 32066449 Free PMC article.
-
Dry Powder Comprised of Isoniazid-Loaded Nanoparticles of Hyaluronic Acid in Conjugation with Mannose-Anchored Chitosan for Macrophage-Targeted Pulmonary Administration in Tuberculosis.Pharmaceutics. 2022 Jul 25;14(8):1543. doi: 10.3390/pharmaceutics14081543. Pharmaceutics. 2022. PMID: 35893799 Free PMC article.
-
Development of mannose functionalized dendrimeric nanoparticles for targeted delivery to macrophages: use of this platform to modulate atherosclerosis.Transl Res. 2018 Mar;193:13-30. doi: 10.1016/j.trsl.2017.10.008. Epub 2017 Nov 21. Transl Res. 2018. PMID: 29172034 Free PMC article.
References
-
- Hettinger J, Richards DM, Hansson J, Barra MM, Joschko AC, Krijgsveld J, Feuerer M. Origin of monocytes and macrophages in a committed progenitor. Nature immunology. 2013;14:821–830. - PubMed
-
- Lee KM, Yin C, Verschoor CP, Bowdish DM. Macrophage function disorders. eLS. 2001
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

