Calcium-mediated oxidative stress: a common mechanism in tight junction disruption by different types of cellular stress
- PMID: 28057718
- PMCID: PMC5723146
- DOI: 10.1042/BCJ20160679
Calcium-mediated oxidative stress: a common mechanism in tight junction disruption by different types of cellular stress
Abstract
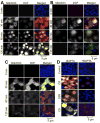
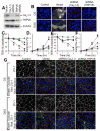
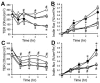
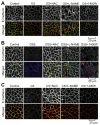
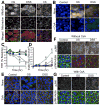
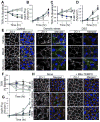
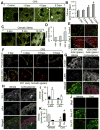
The role of reactive oxygen species (ROS) in osmotic stress, dextran sulfate sodium (DSS) and cyclic stretch-induced tight junction (TJ) disruption was investigated in Caco-2 cell monolayers in vitro and restraint stress-induced barrier dysfunction in mouse colon in vivo Live cell imaging showed that osmotic stress, cyclic stretch and DSS triggered rapid production of ROS in Caco-2 cell monolayers, which was blocked by depletion of intracellular Ca2+ by 1,2-bis-(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid. Knockdown of CaV1.3 or TRPV6 channels blocked osmotic stress and DSS-induced ROS production and attenuated TJ disruption and barrier dysfunction. N-Acetyl l-cysteine (NAC) and l-NG-Nitroarginine methyl ester (l-NAME) blocked stress-induced TJ disruption and barrier dysfunction. NAC and l-NAME also blocked stress-induced activation of c-Jun N-terminal kinase (JNK) and c-Src. ROS was colocalized with the mitochondrial marker in stressed cells. Cyclosporin A blocked osmotic stress and DSS-induced ROS production, barrier dysfunction, TJ disruption and JNK activation. Mitochondria-targeted Mito-TEMPO blocked osmotic stress and DSS-induced barrier dysfunction and TJ disruption. Chronic restraint stress in mice resulted in the elevation of intracellular Ca2+, activation of JNK and c-Src, and disruption of TJ in the colonic epithelium. Furthermore, corticosterone administration induced JNK and c-Src activation, TJ disruption and protein thiol oxidation in colonic mucosa. The present study demonstrates that oxidative stress is a common signal in the mechanism of TJ disruption in the intestinal epithelium by different types of cellular stress in vitro and bio behavioral stress in vivo.
Keywords: ROS; adherens junction; barrier function; epithelium; intestine; tight junction.
© 2017 The Author(s); published by Portland Press Limited on behalf of the Biochemical Society.
Figures


Similar articles
-
Calcium/Ask1/MKK7/JNK2/c-Src signalling cascade mediates disruption of intestinal epithelial tight junctions by dextran sulfate sodium.Biochem J. 2015 Feb 1;465(3):503-15. doi: 10.1042/BJ20140450. Biochem J. 2015. PMID: 25377781 Free PMC article.
-
CaV1.3 channels and intracellular calcium mediate osmotic stress-induced N-terminal c-Jun kinase activation and disruption of tight junctions in Caco-2 CELL MONOLAYERS.J Biol Chem. 2011 Aug 26;286(34):30232-43. doi: 10.1074/jbc.M111.240358. Epub 2011 Jul 7. J Biol Chem. 2011. PMID: 21737448 Free PMC article.
-
Calcium Channels and Oxidative Stress Mediate a Synergistic Disruption of Tight Junctions by Ethanol and Acetaldehyde in Caco-2 Cell Monolayers.Sci Rep. 2016 Dec 13;6:38899. doi: 10.1038/srep38899. Sci Rep. 2016. PMID: 27958326 Free PMC article.
-
Barrier dysfunction in the nasal allergy.Allergol Int. 2018 Jan;67(1):18-23. doi: 10.1016/j.alit.2017.10.006. Epub 2017 Nov 14. Allergol Int. 2018. PMID: 29150353 Review.
-
Tight junction and kidney stone disease.Tissue Barriers. 2024 Jan 2;12(1):2210051. doi: 10.1080/21688370.2023.2210051. Epub 2023 May 10. Tissue Barriers. 2024. PMID: 37162265 Free PMC article. Review.
Cited by
-
The mitochondrial UPR regulator ATF5 promotes intestinal barrier function via control of the satiety response.Cell Rep. 2022 Dec 13;41(11):111789. doi: 10.1016/j.celrep.2022.111789. Cell Rep. 2022. PMID: 36516750 Free PMC article.
-
Pentose Pathway Activation Is Superior to Increased Glycolysis for Therapeutic Angiogenesis in Peripheral Arterial Disease.J Am Heart Assoc. 2023 Apr 4;12(7):e027986. doi: 10.1161/JAHA.122.027986. Epub 2023 Mar 28. J Am Heart Assoc. 2023. PMID: 36974760 Free PMC article.
-
Role of C-Jun N-Terminal Kinases on a Stressed Epithelium: Time for Testing Isoform Specificity.Biology (Basel). 2025 Jun 3;14(6):649. doi: 10.3390/biology14060649. Biology (Basel). 2025. PMID: 40563900 Free PMC article. Review.
-
Panose prevents acute-on-chronic liver failure by reducing bacterial infection in mice.J Clin Invest. 2025 Jun 3;135(14):e184653. doi: 10.1172/JCI184653. eCollection 2025 Jul 15. J Clin Invest. 2025. PMID: 40478727 Free PMC article.
-
Oxalate induces type II epithelial to mesenchymal transition (EMT) in inner medullary collecting duct cells (IMCD) in vitro and stimulate the expression of osteogenic and fibrotic markers in kidney medulla in vivo.Oncotarget. 2019 Feb 1;10(10):1102-1118. doi: 10.18632/oncotarget.26634. eCollection 2019 Feb 1. Oncotarget. 2019. PMID: 30800221 Free PMC article.
References
-
- Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. 2011;62:591–599. - PubMed
-
- Jager S, Stange EF, Wehkamp J. Inflammatory bowel disease: an impaired barrier disease. Langenbecks Arch Surg. 2013;398:1–12. - PubMed
-
- Cameron HL, Perdue MH. Stress impairs murine intestinal barrier function: improvement by glucagon-like peptide-2. J Pharmacol Exp Ther. 2005;314:214–220. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

