Selection and stabilization of endocytic sites by Ede1, a yeast functional homologue of human Eps15
- PMID: 28057762
- PMCID: PMC5328616
- DOI: 10.1091/mbc.E16-06-0391
Selection and stabilization of endocytic sites by Ede1, a yeast functional homologue of human Eps15
Abstract
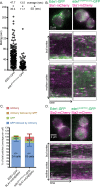
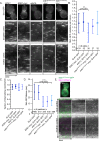
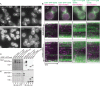
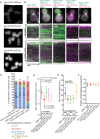
During clathrin-mediated endocytosis (CME), endocytic-site maturation can be divided into two stages corresponding to the arrival of the early and late proteins at the plasma membrane. The early proteins are required to capture cargo and position the late machinery, which includes proteins involved in actin assembly and membrane scission. However, the mechanism by which early-arriving proteins select and stabilize endocytic sites is not known. Ede1, one of the earliest proteins recruited to endocytic sites, facilitates site initiation and stabilization. Deletion of EDE1 results in fewer CME initiations and defects in the timing of vesicle maturation. Here we made truncation mutants of Ede1 to better understand how different domains contribute to its recruitment to CME sites, site selection, and site maturation. We found that the minimal domains required for efficient Ede1 localization at CME sites are the third EH domain, the proline-rich region, and the coiled-coil region. We also found that many strains expressing ede1 truncations could support a normal rate of site initiation but still had defects in site-maturation timing, indicating separation of Ede1 functions. When expressed in yeast, human Eps15 localized to the plasma membrane, where it recruited late-phase CME proteins and supported productive endocytosis, identifying it as an Ede1 functional homologue.
© 2017 Lu and Drubin. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures

References
-
- Brach T, Godlee C, Moeller-Hansen I, Boeke D, Kaksonen M. The initiation of clathrin-mediated endocytosis is mechanistically highly flexible. Curr Biol. 2014;24:548–554. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

