Insulin signaling regulates a functional interaction between adenomatous polyposis coli and cytoplasmic dynein
- PMID: 28057765
- PMCID: PMC5328618
- DOI: 10.1091/mbc.E16-07-0555
Insulin signaling regulates a functional interaction between adenomatous polyposis coli and cytoplasmic dynein
Abstract
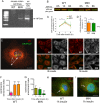
Diabetes is linked to an increased risk for colorectal cancer, but the mechanistic underpinnings of this clinically important effect are unclear. Here we describe an interaction between the microtubule motor cytoplasmic dynein, the adenomatous polyposis coli tumor suppressor protein (APC), and glycogen synthase kinase-3β (GSK-3β), which could shed light on this issue. GSK-3β is perhaps best known for glycogen regulation, being inhibited downstream in an insulin-signaling pathway. However, the kinase is also important in many other processes. Mutations in APC that disrupt the regulation of β-catenin by GSK-3β cause colorectal cancer in humans. Of interest, both APC and GSK-3β interact with microtubules and cellular membranes. We recently demonstrated that dynein is a GSK-3β substrate and that inhibition of GSK-3β promotes dynein-dependent transport. We now report that dynein stimulation in intestinal cells in response to acute insulin exposure (or GSK-3β inhibition) is blocked by tumor-promoting isoforms of APC that reduce an interaction between wild-type APC and dynein. We propose that under normal conditions, insulin decreases dynein binding to APC to stimulate minus end-directed transport, which could modulate endocytic and secretory systems in intestinal cells. Mutations in APC likely impair the ability to respond appropriately to insulin signaling. This is exciting because it has the potential to be a contributing factor in the development of colorectal cancer in patients with diabetes.
© 2017 Gao, Shi, et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures






References
-
- Ait-Omar A, Monteiro-Sepulveda M, Poitou C, Le Gall M, Cotillard A, Gilet J, Garbin K, Houllier A, Chateau D, Lacombe A, et al. GLUT2 accumulation in enterocyte apical and intracellular membranes: a study in morbidly obese human subjects and ob/ob and high fat-fed mice. Diabetes. 2011;60:2598–2607. - PMC - PubMed
-
- Allan VJ. Cytoplasmic dynein. Biochem Soc Trans. 2011;39:1169–1178. - PubMed
-
- Andres SF, Santoro MA, Mah AT, Keku JA, Bortvedt AE, Blue RE, Lund PK. Deletion of intestinal epithelial insulin receptor attenuates high-fat diet-induced elevations in cholesterol and stem, enteroendocrine, and Paneth cell mRNAs. Am J Physiol Gastrointest Liver Physiol. 2015;308:G100–G111. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

