Extra-nuclear effects of estrogen on cortical bone in males require ERαAF-1
- PMID: 28057769
- PMCID: PMC5278601
- DOI: 10.1530/JME-16-0209
Extra-nuclear effects of estrogen on cortical bone in males require ERαAF-1
Abstract
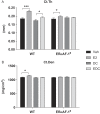
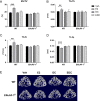
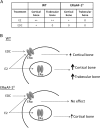
Estradiol (E2) signaling via estrogen receptor alpha (ERα) is important for the male skeleton as demonstrated by ERα inactivation in both mice and man. ERα mediates estrogenic effects not only by translocating to the nucleus and affecting gene transcription but also by extra-nuclear actions e.g., triggering cytoplasmic signaling cascades. ERα contains various domains, and the role of activation function 1 (ERαAF-1) is known to be tissue specific. The aim of this study was to determine the importance of extra-nuclear estrogen effects for the skeleton in males and to determine the role of ERαAF-1 for mediating these effects. Five-month-old male wild-type (WT) and ERαAF-1-inactivated (ERαAF-10) mice were orchidectomized and treated with equimolar doses of 17β-estradiol (E2) or an estrogen dendrimer conjugate (EDC), which is incapable of entering the nucleus and thereby only initiates extra-nuclear ER actions or their corresponding vehicles for 3.5 weeks. As expected, E2 treatment increased cortical thickness and trabecular bone volume per total volume (BV/TV) in WT males. EDC treatment increased cortical thickness in WT males, whereas no effect was detected in trabecular bone. In ERαAF-10 males, E2 treatment increased cortical thickness, but did not affect trabecular bone. Interestingly, the effect of EDC on cortical bone was abolished in ERαAF-10 mice. In conclusion, extra-nuclear estrogen signaling affects cortical bone mass in males, and this effect is dependent on a functional ERαAF-1. Increased knowledge regarding estrogen signaling mechanisms in the regulation of the male skeleton may aid the development of new treatment options for male osteoporosis.
Keywords: cortical bone; estrogen; estrogen dendrimer conjugate; male skeleton.
© 2017 The authors.
Figures
References
-
- Almeida M, Han L, Ambrogini E, Bartell SM, Manolagas SC. 2010. Oxidative stress stimulates apoptosis and activates NF-kappaB in osteoblastic cells via a PKCbeta/p66shc signaling cascade: counter regulation by estrogens or androgens. Molecular Endocrinology 24 2030–2037. ( 10.1210/me.2010-0189) - DOI - PMC - PubMed
-
- Bartell SM, Han L, Kim HN, Kim SH, Katzenellenbogen JA, Katzenellenbogen BS, Chambliss KL, Shaul PW, Roberson PK, Weinstein RS, et al. 2013. Non-nuclear-initiated actions of the estrogen receptor protect cortical bone mass. Molecular Endocrinology 27 649–656. ( 10.1210/me.2012-1368) - DOI - PMC - PubMed
-
- Billon-Gales A, Fontaine C, Filipe C, Douin-Echinard V, Fouque MJ, Flouriot G, Gourdy P, Lenfant F, Laurell H, Krust A, et al. 2009. The transactivating function 1 of estrogen receptor alpha is dispensable for the vasculoprotective actions of 17beta-estradiol. PNAS 106 2053–2058. ( 10.1073/pnas.0808742106) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

