Olefin Isomers of a Triazole Bisphosphonate Synergistically Inhibit Geranylgeranyl Diphosphate Synthase
- PMID: 28057800
- PMCID: PMC5325083
- DOI: 10.1124/mol.116.107326
Olefin Isomers of a Triazole Bisphosphonate Synergistically Inhibit Geranylgeranyl Diphosphate Synthase
Abstract
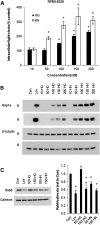
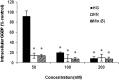
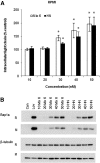
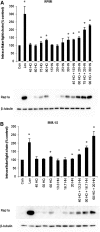
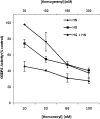
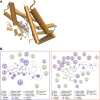
The isoprenoid donor for protein geranylgeranylation reactions, geranylgeranyl diphosphate (GGDP), is the product of the enzyme GGDP synthase (GGDPS) that condenses farnesyl diphosphate (FDP) and isopentenyl pyrophosphate. GGDPS inhibition is of interest from a therapeutic perspective for multiple myeloma because we have shown that targeting Rab GTPase geranylgeranylation impairs monoclonal protein trafficking, leading to endoplasmic reticulum stress and apoptosis. We reported a series of triazole bisphosphonate GGDPS inhibitors, of which the most potent was a 3:1 mixture of homogeranyl (HG) and homoneryl (HN) isomers. Here we determined the activity of the individual olefin isomers. Enzymatic and cellular assays revealed that although HN is approximately threefold more potent than HG, HN is not more potent than the original mixture. Studies in which cells were treated with varying concentrations of each isomer alone and in different combinations revealed that the two isomers potentiate the induced-inhibition of protein geranylgeranylation when used in a 3:1 HG:HN combination. A synergistic interaction was observed between the two isomers in the GGDPS enzyme assay. These results suggested that the two isomers bind simultaneously to the enzyme but within different domains. Computational modeling studies revealed that HN is preferred at the FDP site, that HG is preferred at the GGDP site, and that both isomers may bind to the enzyme simultaneously. These studies are the first to report a set of olefin isomers that synergistically inhibit GGDPS, thus establishing a new paradigm for the future development of GGDPS inhibitors.
Copyright © 2017 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


References
-
- Barney RJ, Wasko BM, Dudakovic A, Hohl RJ, Wiemer DF. (2010) Synthesis and biological evaluation of a series of aromatic bisphosphonates. Bioorg Med Chem 18:7212–7220. - PubMed
-
- Bergstrom JD, Bostedor RG, Masarachia PJ, Reszka AA, Rodan G. (2000) Alendronate is a specific, nanomolar inhibitor of farnesyl diphosphate synthase. Arch Biochem Biophys 373:231–241. - PubMed
-
- Chou TC, Talalay P. (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55. - PubMed
-
- Coxon FP, Helfrich MH, Van’t Hof R, Sebti S, Ralston SH, Hamilton A, Rogers MJ. (2000) Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res 15:1467–1476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

