A serine/threonine phosphatase 1 of Streptococcus suis type 2 is an important virulence factor
- PMID: 28057904
- PMCID: PMC5746436
- DOI: 10.4142/jvs.2017.18.4.439
A serine/threonine phosphatase 1 of Streptococcus suis type 2 is an important virulence factor
Abstract
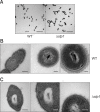
Streptococcus suis is regarded as one of the major pathogens of pigs, and Streptococcus suis type 2 (SS2) is considered a zoonotic bacterium based on its ability to cause meningitis and streptococcal toxic shock-like syndrome in humans. Many bacterial species contain genes encoding serine/threonine protein phosphatases (STPs) responsible for dephosphorylation of their substrates in a single reaction step. This study investigated the role of stp1 in the pathogenesis of SS2. An isogenic stp1 mutant (Δstp1) was constructed from SS2 strain ZJ081101. The Δstp1 mutant exhibited a significant increase in adhesion to HEp-2 and bEnd.3 cells as well as increased survival in RAW264.7 cells, as compared to the parent strain. Increased survival in macrophage cells might be related to resistance to reactive oxygen species since the Δstp1 mutant was more resistant than its parent strain to paraquat-induced oxidative stress. However, compared to parent strain virulence, deletion of stp1 significantly attenuated virulence of SS2 in mice, as shown by the nearly double lethal dose 50 value and the lower bacterial load in organs and blood in the murine model. We conclude that Stp1 has an essential role in SS2 virulence.
Keywords: Streptococcus suis type 2; serine/threonine protein phosphatase; virulence.
Conflict of interest statement
Figures




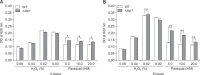

Similar articles
-
Inactivation of the sodA gene of Streptococcus suis type 2 encoding superoxide dismutase leads to reduced virulence to mice.Vet Microbiol. 2012 Aug 17;158(3-4):360-6. doi: 10.1016/j.vetmic.2012.02.028. Epub 2012 Feb 28. Vet Microbiol. 2012. PMID: 22424868
-
GntR is involved in the expression of virulence in strain Streptococcus suis P1/7.FEMS Microbiol Lett. 2018 Jul 1;365(14). doi: 10.1093/femsle/fny091. FEMS Microbiol Lett. 2018. PMID: 29635445
-
Stk and Stp1 participate in Streptococcus suis serotype 2 pathogenesis by regulating capsule thickness and translocation of certain virulence factors.Microb Pathog. 2021 Mar;152:104607. doi: 10.1016/j.micpath.2020.104607. Epub 2020 Nov 5. Microb Pathog. 2021. PMID: 33161059
-
Uncovering newly emerging variants of Streptococcus suis, an important zoonotic agent.Trends Microbiol. 2010 Mar;18(3):124-31. doi: 10.1016/j.tim.2009.12.003. Epub 2010 Jan 12. Trends Microbiol. 2010. PMID: 20071175 Review.
-
Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis.Future Microbiol. 2012 Feb;7(2):259-79. doi: 10.2217/fmb.11.149. Future Microbiol. 2012. PMID: 22324994 Review.
Cited by
-
Involvement of Various Enzymes in the Physiology and Pathogenesis of Streptococcus suis.Vet Sci. 2020 Sep 23;7(4):143. doi: 10.3390/vetsci7040143. Vet Sci. 2020. PMID: 32977655 Free PMC article. Review.
-
Streptococcus suis DivIVA Protein Is a Substrate of Ser/Thr Kinase STK and Involved in Cell Division Regulation.Front Cell Infect Microbiol. 2018 Mar 20;8:85. doi: 10.3389/fcimb.2018.00085. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29616196 Free PMC article.
-
The Redox-Sensing Regulator Rex Contributes to the Virulence and Oxidative Stress Response of Streptococcus suis Serotype 2.Front Cell Infect Microbiol. 2018 Sep 18;8:317. doi: 10.3389/fcimb.2018.00317. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30280091 Free PMC article.
-
Mechanisms governing bacterial capsular polysaccharide attachment and chain length.Ann N Y Acad Sci. 2025 Jun;1548(1):80-98. doi: 10.1111/nyas.15364. Epub 2025 May 14. Ann N Y Acad Sci. 2025. PMID: 40369709 Free PMC article. Review.
-
Comparative Phenotypic, Proteomic, and Phosphoproteomic Analysis Reveals Different Roles of Serine/Threonine Phosphatase and Kinase in the Growth, Cell Division, and Pathogenicity of Streptococcus suis.Microorganisms. 2021 Nov 26;9(12):2442. doi: 10.3390/microorganisms9122442. Microorganisms. 2021. PMID: 34946045 Free PMC article.
References
-
- Archambaud C, Nahori MA, Pizarro-Cerda J, Cossart P, Dussurget O. Control of Listeria superoxide dismutase by phosphorylation. J Biol Chem. 2006;281:31812–31822. - PubMed
-
- Barford D, Das AK, Egloff MP. The structure and mechanism of protein phosphatases: insights into catalysis and regulation. Annu Rev Biophys Biomol Struct. 1998;27:133–164. - PubMed
-
- Baums CG, Valentin-Weigand P. Surface-associated and secreted factors of Streptococcus suis in epidemiology, pathogenesis and vaccine development. Anim Health Res Rev. 2009;10:65–83. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

