Activating JAK2 mutants reveal cytokine receptor coupling differences that impact outcomes in myeloproliferative neoplasm
- PMID: 28057939
- PMCID: PMC5589508
- DOI: 10.1038/leu.2017.1
Activating JAK2 mutants reveal cytokine receptor coupling differences that impact outcomes in myeloproliferative neoplasm
Abstract
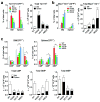
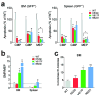
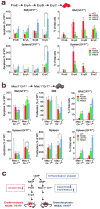
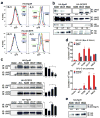
Janus tyrosine kinase 2 (JAK2) mediates downstream signaling of cytokine receptors in all hematological lineages, yet constitutively active JAK2 mutants are able to drive selective expansion of particular lineage(s) in myeloproliferative neoplasm (MPN). The molecular basis of lineage specificity is unclear. Here, we show that three activating JAK2 mutants with similar kinase activities in vitro elicit distinctive MPN phenotypes in mice by differentially expanding erythroid vs granulocytic precursors. Molecularly, this reflects the differential binding of JAK2 mutants to cytokine receptors EpoR and GCSFR in the erythroid vs granulocytic lineage and the creation of unique receptor/JAK2 complexes that generate qualitatively distinct downstream signals. Our results demonstrate that activating JAK2 mutants can differentially couple to selective cytokine receptors and change the signaling repertoire, revealing the molecular basis for phenotypic differences elicited by JAK2 (V617F) or mutations in exon 12. On the basis of these findings, receptor-JAK2 interactions could represent new targets of lineage-specific therapeutic approaches against MPN, which may be applicable to other cancers with aberrant JAK-STAT signaling.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Figures


Similar articles
-
JAK2, the JAK2 V617F mutant and cytokine receptors.Pathol Biol (Paris). 2007 Mar;55(2):88-91. doi: 10.1016/j.patbio.2006.06.003. Epub 2006 Aug 14. Pathol Biol (Paris). 2007. PMID: 16904848 Review.
-
Three Tyrosine Residues in the Erythropoietin Receptor Are Essential for Janus Kinase 2 V617F Mutant-induced Tumorigenesis.J Biol Chem. 2017 Feb 3;292(5):1826-1846. doi: 10.1074/jbc.M116.749465. Epub 2016 Dec 20. J Biol Chem. 2017. PMID: 27998978 Free PMC article.
-
HLA-G turns off erythropoietin receptor signaling through JAK2 and JAK2 V617F dephosphorylation: clinical relevance in polycythemia vera.Leukemia. 2008 Mar;22(3):578-84. doi: 10.1038/sj.leu.2405050. Epub 2007 Dec 6. Leukemia. 2008. PMID: 18059484
-
ATP binding to the pseudokinase domain of JAK2 is critical for pathogenic activation.Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):4642-7. doi: 10.1073/pnas.1423201112. Epub 2015 Mar 30. Proc Natl Acad Sci U S A. 2015. PMID: 25825724 Free PMC article.
-
[Analysis of oncogenic signaling pathway induced by a myeloproliferative neoplasm-associated Janus kinase 2 (JAK2) V617F mutant].Yakugaku Zasshi. 2012;132(11):1267-72. doi: 10.1248/yakushi.12-00225. Yakugaku Zasshi. 2012. PMID: 23123718 Review. Japanese.
Cited by
-
The Role of PI3K/AKT and MAPK Signaling Pathways in Erythropoietin Signalization.Int J Mol Sci. 2021 Jul 19;22(14):7682. doi: 10.3390/ijms22147682. Int J Mol Sci. 2021. PMID: 34299300 Free PMC article. Review.
-
Molecular basis of JAK2 activation in erythropoietin receptor and pathogenic JAK2 signaling.Sci Adv. 2024 Mar 8;10(10):eadl2097. doi: 10.1126/sciadv.adl2097. Epub 2024 Mar 8. Sci Adv. 2024. PMID: 38457493 Free PMC article.
-
Epo-IGF1R cross talk expands stress-specific progenitors in regenerative erythropoiesis and myeloproliferative neoplasm.Blood. 2022 Dec 1;140(22):2371-2384. doi: 10.1182/blood.2022016741. Blood. 2022. PMID: 36054916 Free PMC article.
-
Experimental Modeling of Myeloproliferative Neoplasms.Genes (Basel). 2019 Oct 15;10(10):813. doi: 10.3390/genes10100813. Genes (Basel). 2019. PMID: 31618985 Free PMC article. Review.
-
MALAT1/miR-582-5p/GALNT1/MUC1 axis modulates progression of AML leukemia stem cells by regulating JAK2/STAT3 pathway.Ann Hematol. 2024 Dec;103(12):5273-5283. doi: 10.1007/s00277-024-06043-w. Epub 2024 Oct 21. Ann Hematol. 2024. PMID: 39428449
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

