Vitamin D differentially regulates Salmonella-induced intestine epithelial autophagy and interleukin-1β expression
- PMID: 28058015
- PMCID: PMC5175247
- DOI: 10.3748/wjg.v22.i47.10353
Vitamin D differentially regulates Salmonella-induced intestine epithelial autophagy and interleukin-1β expression
Abstract
Aim: To investigate the effects of active vitamin D3 on autophagy and interleukin (IL)-1β expression in Salmonella-infected intestinal epithelial cells (IECs).
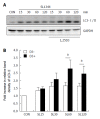
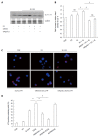
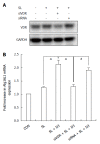
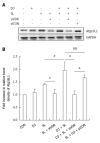
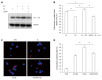
Methods: Caco-2 cells, NOD2 siRNA-, Atg16L1 siRNA- or vitamin D receptor (VDR) siRNA-transfected Caco-2 cells were pretreated with 1,25-dihydroxyvitamin D3 (1,25D3), and then infected by wild-type S. typhimurium strain SL1344. The conversion of LC3-I to LC3-II was detected by Western blot analysis and LC3+ autophagosome was analyzed by immunofluorescence. Caco-2 cells or VDR siRNA-transfected cells were pretreated with 1,25D3, and then infected by SL1344. Membrane protein and total RNA were analyzed by Western blot and RT-PCR for VDR and Atg16L1 protein and mRNA expression, respectively. Atg16L1 siRNA-transfected Caco-2 cells were pretreated by 1,25D3 and then infected with SL1344. Total RNA was analyzed by RT-PCR for IL-1β mRNA expression.
Results: The active form of vitamin D, 1,25D3, showed enhanced VDR-mediated Atg16L1 mRNA expression, membranous Atg16L1 protein expression leading to enhanced autophagic LC3II protein expression and LC3 punctae in Salmonella-infected Caco-2 cells which was counteracted by Atg16L1 and VDR siRNA, but Atg16L1 mediated suppression of IL-1β expression. Thus, active vitamin D may enhance autophagy but suppress inflammatory IL-1β expression in Salmonella-infected IECs.
Conclusion: Active vitamin D might enhance autophagic clearance of Salmonella infection, while modulation of inflammatory responses prevents the host from detrimental effects of overwhelming inflammation.
Keywords: Atg16L1; Autophagy; Interleukin-1β; Intestinal epithelia; Salmonella; Vitamin D.
Conflict of interest statement
Conflict-of-interest statement: The author declares that there are no financial or commercial conflicts of interest.
Figures


References
-
- Glynn MK, Bopp C, Dewitt W, Dabney P, Mokhtar M, Angulo FJ. Emergence of multidrug-resistant Salmonella enterica serotype typhimurium DT104 infections in the United States. N Engl J Med. 1998;338:1333–1338. - PubMed
-
- Parry CM. Antimicrobial drug resistance in Salmonella enterica. Curr Opin Infect Dis. 2003;16:467–472. - PubMed
-
- Lauderdale TL, Aarestrup FM, Chen PC, Lai JF, Wang HY, Shiau YR, Huang IW, Hung CL. Multidrug resistance among different serotypes of clinical Salmonella isolates in Taiwan. Diagn Microbiol Infect Dis. 2006;55:149–155. - PubMed
-
- Nicholson I, Dalzell AM, El-Matary W. Vitamin D as a therapy for colitis: a systematic review. J Crohns Colitis. 2012;6:405–411. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

