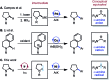
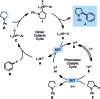
C-H functionalization of amines with aryl halides by nickel-photoredox catalysis
- PMID: 28058105
- PMCID: PMC5207500
- DOI: 10.1039/C6SC02815B
C-H functionalization of amines with aryl halides by nickel-photoredox catalysis
Abstract
We describe the functionalization of α-amino C-H bonds with aryl halides using a combination of nickel and photoredox catalysis. This direct C-H, C-X coupling uses inexpensive and readily available starting materials to generate benzylic amines, an important class of bioactive molecules. Mechanistically, this method features the direct arylation of α-amino radicals mediated by a nickel catalyst. This reactivity is demonstrated for a range of aryl halides and N-aryl amines, with orthogonal scope to existing C-H activation and photoredox methodologies. We also report reactions with several complex aryl halides, demonstrating the potential utility of this approach in late-stage functionalization.
Figures
Similar articles
-
Synthetic and Mechanistic Implications of Chlorine Photoelimination in Nickel/Photoredox C(sp3)-H Cross-Coupling.Acc Chem Res. 2021 Feb 16;54(4):988-1000. doi: 10.1021/acs.accounts.0c00694. Epub 2021 Jan 29. Acc Chem Res. 2021. PMID: 33511841 Free PMC article.
-
Modular Access to Chiral α-(Hetero)aryl Amines via Ni/Photoredox-Catalyzed Enantioselective Cross-Coupling.J Am Chem Soc. 2022 May 18;144(19):8797-8806. doi: 10.1021/jacs.2c02795. Epub 2022 May 3. J Am Chem Soc. 2022. PMID: 35503417
-
Direct Acylation of C(sp(3))-H Bonds Enabled by Nickel and Photoredox Catalysis.Angew Chem Int Ed Engl. 2016 Mar 14;55(12):4040-3. doi: 10.1002/anie.201511438. Epub 2016 Feb 17. Angew Chem Int Ed Engl. 2016. PMID: 26890705 Free PMC article.
-
Photoredox-Catalyzed C-H Functionalization Reactions.Chem Rev. 2022 Jan 26;122(2):1925-2016. doi: 10.1021/acs.chemrev.1c00311. Epub 2021 Sep 29. Chem Rev. 2022. PMID: 34585909 Free PMC article. Review.
-
Orthogonal Nanoparticle Catalysis with Organogermanes.Angew Chem Int Ed Engl. 2019 Dec 2;58(49):17788-17795. doi: 10.1002/anie.201910060. Epub 2019 Oct 23. Angew Chem Int Ed Engl. 2019. PMID: 31562670 Free PMC article. Review.
Cited by
-
Regio- and chemoselective Csp3-H arylation of benzylamines by single electron transfer/hydrogen atom transfer synergistic catalysis.Chem Sci. 2018 Sep 12;9(44):8453-8460. doi: 10.1039/c8sc02965b. eCollection 2018 Nov 28. Chem Sci. 2018. PMID: 30542595 Free PMC article.
-
Photoredox Radical/Polar Crossover Enables Construction of Saturated Nitrogen Heterocycles.Org Lett. 2019 Apr 5;21(7):2317-2321. doi: 10.1021/acs.orglett.9b00602. Epub 2019 Mar 12. Org Lett. 2019. PMID: 30860849 Free PMC article.
-
Reactivity of (bi-Oxazoline)organonickel Complexes and Revision of a Catalytic Mechanism.J Am Chem Soc. 2021 Sep 15;143(36):14458-14463. doi: 10.1021/jacs.1c07139. Epub 2021 Aug 31. J Am Chem Soc. 2021. PMID: 34463481 Free PMC article.
-
Direct C(sp3)-H Cross Coupling Enabled by Catalytic Generation of Chlorine Radicals.J Am Chem Soc. 2016 Oct 5;138(39):12719-12722. doi: 10.1021/jacs.6b08397. Epub 2016 Sep 21. J Am Chem Soc. 2016. PMID: 27653738 Free PMC article.
-
Arylboration of Enecarbamates for the Synthesis of Borylated Saturated N-Heterocycles.Angew Chem Int Ed Engl. 2022 Nov 14;61(46):e202212117. doi: 10.1002/anie.202212117. Epub 2022 Oct 17. Angew Chem Int Ed Engl. 2022. PMID: 36250954 Free PMC article.
References
-
- Jazzar R., Hitce J., Renaudat A., Sofack-Kreutzer J., Baudoin O. Chem.–Eur. J. 2010;16:2654–2672. - PubMed
- Baudoin O. Chem. Soc. Rev. 2011;40:4902–4911. - PubMed
- Ackermann L. Chem. Rev. 2011;111:1315–1345. - PubMed
- Daugulis O., Do H.-Q., Shabashov D. Acc. Chem. Res. 2009;42:1074–1086. - PMC - PubMed
- Lyons T. W., Sanford M. S. Chem. Rev. 2010;110:1147–1169. - PMC - PubMed
- Chen X., Engle K. M., Wang D.-H., Yu J.-Q. Angew. Chem., Int. Ed. 2009;48:5094–5115. - PMC - PubMed
- Kakiuchi F., Kochi T. Synthesis. 2008;19:2013–3039.
- Bellina F., Rossi R. Chem. Rev. 2010;110:1082–1146. - PubMed
-
- Lawrence S. A., Amines: Synthesis, Properties and Applications, Cambridge University Press, Cambridge, U.K, 2004.
- Chiral Amine Synthesis: Methods, Developments and Applications, ed. T. C. Nugent, Wiley-VCH: Weinheim, Germany, 2010.
-
- Pastine S. J., Gribkov D. V., Sames D. J. Am. Chem. Soc. 2006;128:14220–14221. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources