C-H functionalization of amines with aryl halides by nickel-photoredox catalysis
- PMID: 28058105
- PMCID: PMC5207500
- DOI: 10.1039/C6SC02815B
C-H functionalization of amines with aryl halides by nickel-photoredox catalysis
Abstract
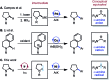
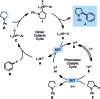
We describe the functionalization of α-amino C-H bonds with aryl halides using a combination of nickel and photoredox catalysis. This direct C-H, C-X coupling uses inexpensive and readily available starting materials to generate benzylic amines, an important class of bioactive molecules. Mechanistically, this method features the direct arylation of α-amino radicals mediated by a nickel catalyst. This reactivity is demonstrated for a range of aryl halides and N-aryl amines, with orthogonal scope to existing C-H activation and photoredox methodologies. We also report reactions with several complex aryl halides, demonstrating the potential utility of this approach in late-stage functionalization.
Figures
References
-
- Jazzar R., Hitce J., Renaudat A., Sofack-Kreutzer J., Baudoin O. Chem.–Eur. J. 2010;16:2654–2672. - PubMed
- Baudoin O. Chem. Soc. Rev. 2011;40:4902–4911. - PubMed
- Ackermann L. Chem. Rev. 2011;111:1315–1345. - PubMed
- Daugulis O., Do H.-Q., Shabashov D. Acc. Chem. Res. 2009;42:1074–1086. - PMC - PubMed
- Lyons T. W., Sanford M. S. Chem. Rev. 2010;110:1147–1169. - PMC - PubMed
- Chen X., Engle K. M., Wang D.-H., Yu J.-Q. Angew. Chem., Int. Ed. 2009;48:5094–5115. - PMC - PubMed
- Kakiuchi F., Kochi T. Synthesis. 2008;19:2013–3039.
- Bellina F., Rossi R. Chem. Rev. 2010;110:1082–1146. - PubMed
-
- Lawrence S. A., Amines: Synthesis, Properties and Applications, Cambridge University Press, Cambridge, U.K, 2004.
- Chiral Amine Synthesis: Methods, Developments and Applications, ed. T. C. Nugent, Wiley-VCH: Weinheim, Germany, 2010.
-
- Pastine S. J., Gribkov D. V., Sames D. J. Am. Chem. Soc. 2006;128:14220–14221. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources