Nickel-catalyzed enantioselective arylation of pyridine
- PMID: 28058106
- PMCID: PMC5207490
- DOI: 10.1039/c6sc00702c
Nickel-catalyzed enantioselective arylation of pyridine
Abstract
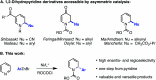
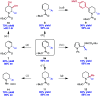
We report an enantioselective Ni-catalyzed cross coupling of arylzinc reagents with pyridiniumions formed in situ from pyridine and a chloroformate. This reaction provides enantioenriched 2-aryl-1,2-dihydropyridine products that can be elaborated to numerous piperidine derivatives with little or no loss in ee. This method is notable for its use of pyridine, a feedstock chemical, to build a versatile, chiral heterocycle in a single synthetic step.
Figures
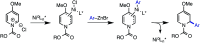

References
-
-
For reviews on the synthesis of dihydropyridines, see:
- Eisner U. Kuthan J. Chem. Rev. 1972;72:1. doi: 10.1021/cr60275a001. - DOI
- Stout D. M. Meyers A. I. Chem. Rev. 1982;82:223. doi: 10.1021/cr00048a004. - DOI
- Lavilla R. J. Chem. Soc., Perkin Trans. 1. 2002:1141. doi: 10.1039/B101371H. - DOI
- Bull J. A. Mousseau J. J. Pelletier G. Charette A. B. Chem. Rev. 2012;112:2642. doi: 10.1021/cr200251d. - DOI - PubMed
- Silva E. M. P. Varandas P. A. M. M. Silva A. M. S. Synthesis. 2013;45:3053. doi: 10.1055/s-0033-1338537. - DOI
-
-
-
Selected examples:
- Koike T. Shinohara Y. Tanabe M. Takeuchi N. Tobinaga S. Chem. Pharm. Bull. 1999;47:1246. doi: 10.1248/cpb.47.1246. - DOI
- Tanaka K. Mori H. Yamamoto M. Katsumura S. J. Org. Chem. 2001;66:3099. doi: 10.1021/jo005779+. - DOI - PubMed
- Harschneck T. Kirsch S. F. J. Org. Chem. 2011;76:2145. doi: 10.1021/jo102545m. - DOI - PubMed
- Trost B. M. Biannic B. Org. Lett. 2015;17:1433. doi: 10.1021/acs.orglett.5b00279. - DOI - PMC - PubMed
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

