Hepatocyte Is a Sole Cell Type Responsible for the Production of Coagulation Factor IX In Vivo
- PMID: 28058178
- PMCID: PMC5196924
- DOI: 10.3727/215517912X639496
Hepatocyte Is a Sole Cell Type Responsible for the Production of Coagulation Factor IX In Vivo
Abstract
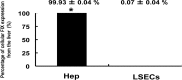
Coagulation factor IX (FIX) is synthesized by hepatocytes, and the lack of this protein causes hemophilia B. Liver nonparenchymal cells, including liver sinusoidal endothelial cells (LSECs) and extrahepatic cells in the body, are scarcely shown to have an ability to synthesize and secrete FIX. The present study investigated the existence of cells responsible for synthesizing FIX other than hepatocytes in mice using gene expression analyses and FIX-specific clotting assays. Among the several organs investigated, including liver, lung, spleen, kidney, brain, intestine, and tongue, FIX mRNA expressions were observed only in the liver. From the liver, hepatocytes and LSECs were isolated. FIX mRNA expression and FIX protein secretion were observed exclusively in the hepatocytes. Furthermore, the clotting activity of FIX secreted from the cultured hepatocytes was found to be dependent on the concentration of vitamin K2. These findings indicated that the hepatocyte is the only cell type that biochemically produces functional FIX in vivo. This highlights the importance of hepatocytes or cells that are fully differentiated toward the hepatic lineage for possible application for regenerative medicine and for targeting gene delivery to establish new cell-based treatments for hemophilia B.
Keywords: Factor IX; Hemophilia B; Hepatocyte; Nonparenchymal cell.
Figures




References
-
- Birraux J.; Menzel O.; Wildhaber B.; Jond C.; Nguyen T. H.; Chardot C. A step toward liver gene therapy: Efficient correction of the genetic defect of hepatocytes isolated from a patient with Crigler-Najjar syndrome type 1 with lentiviral vectors. Transplantation 87:1006–1012;2009. - PubMed
-
- Bolton-Maggs P. H.; Pasi K. J. Haemophilias A and B. Lancet 361:1801– 1809; 2003. - PubMed
-
- Boost K. A.; Auth M. K.; Woitaschek D.; Kim H. S.; Hilgard P.; Nadalin S.; Blaheta R. A. Long-term production of major coagulation factors and inhibitors by primary human hepatocytes in vitro: Perspectives for clinical application. Liver Int. 27:832–844;2007. - PubMed
-
- Coutu D. L.; Cuerquis J.; El Ayoubi R.; Forner K. A.; Roy R.; Francois M.; Griffith M.; Lillicrap D.; Yousefi A. M.; Blostein M. D.; Galipeau J. Hierarchical scaffold design for mesenchymal stem cell-based gene therapy of hemophilia B. Biomaterials 32:295–305;2011. - PubMed
LinkOut - more resources
Full Text Sources

