Remodeling of the Inner Hair Cell Microtubule Meshwork in a Mouse Model of Auditory Neuropathy AUNA1
- PMID: 28058271
- PMCID: PMC5197407
- DOI: 10.1523/ENEURO.0295-16.2016
Remodeling of the Inner Hair Cell Microtubule Meshwork in a Mouse Model of Auditory Neuropathy AUNA1
Abstract
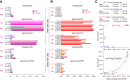
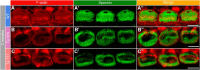
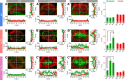
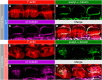
Auditory neuropathy 1 (AUNA1) is a form of human deafness resulting from a point mutation in the 5' untranslated region of the Diaphanous homolog 3 (DIAPH3) gene. Notably, the DIAPH3 mutation leads to the overexpression of the DIAPH3 protein, a formin family member involved in cytoskeleton dynamics. Through study of diap3-overexpressing transgenic (Tg) mice, we examine in further detail the anatomical, functional, and molecular mechanisms underlying AUNA1. We identify diap3 as a component of the hair cells apical pole in wild-type mice. In the diap3-overexpressing Tg mice, which show a progressive threshold shift associated with a defect in inner hair cells (IHCs), the neurotransmitter release and potassium conductances are not affected. Strikingly, the overexpression of diap3 results in a selective and early-onset alteration of the IHC cuticular plate. Molecular dissection of the apical components revealed that the microtubule meshwork first undergoes aberrant targeting into the cuticular plate of Tg IHCs, followed by collapse of the stereociliary bundle, with eventual loss of the IHC capacity to transmit incoming auditory stimuli.
Keywords: cochlea; cuticular plate; deafness; diap3; diaphanous.
Conflict of interest statement
Authors report no conflict of interest.
Figures





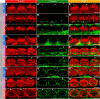
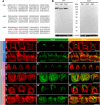
References
-
- Bobbin RP, Fallon M, Kujawa SG (1991) Magnitude of the negative summating potential varies with perilymph calcium levels. Hear Res 56:101–110. - PubMed
-
- Bobbin RP, Jastreboff PJ, Fallon M, Littman T (1990) Nimodipine, an L-channel Ca2+ antagonist, reverses the negative summating potential recorded from the guinea pig cochlea. Hear Res 46:277–287. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
