Synthesis of ZnO/Si Hierarchical Nanowire Arrays for Photocatalyst Application
- PMID: 28058644
- PMCID: PMC5216019
- DOI: 10.1186/s11671-016-1803-0
Synthesis of ZnO/Si Hierarchical Nanowire Arrays for Photocatalyst Application
Abstract
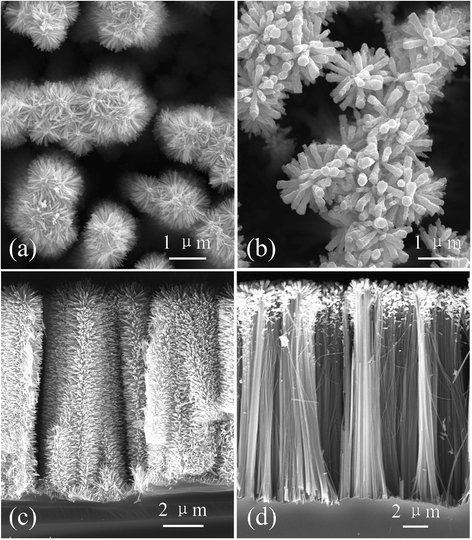
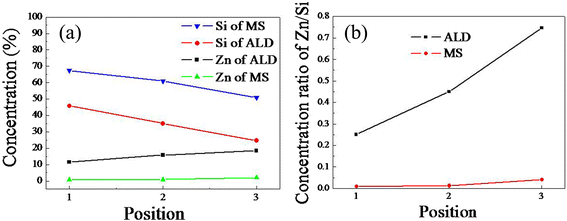
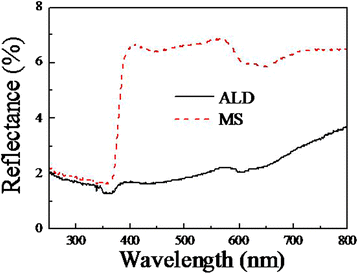
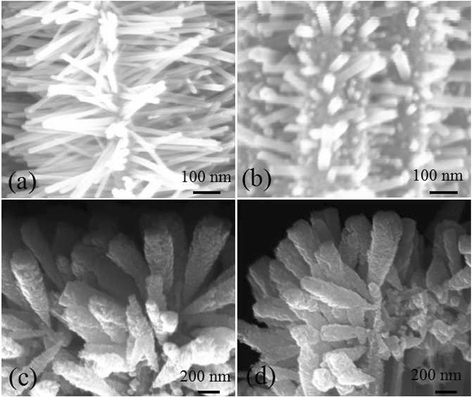
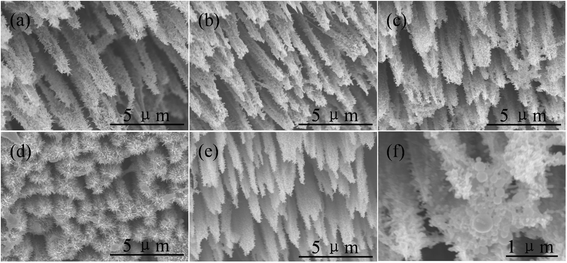
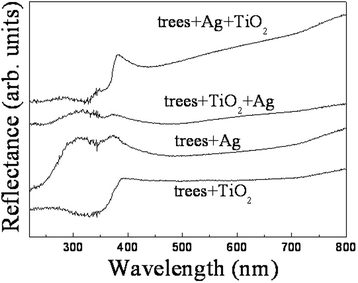
ZnO/Si nanowire arrays with hierarchical architecture were synthesized by solution method with ZnO seed layer grown by atomic layer deposition and magnetron sputtering, respectively. The photocatalytic activity of the as-grown tree-like arrays was evaluated by the degradation of methylene blue under ultraviolet light at ambient temperature. The comparison of morphology, crystal structure, optical properties, and photocatalysis efficiency of the two samples in different seeding processes was conducted. It was found that the ZnO/Si nanowire arrays presented a larger surface area with better crystalline and more uniform ZnO branches on the whole sidewall of Si backbones for the seed layer by atomic layer deposition, which gained a strong light absorption as high as 98% in the ultraviolet and visible range. The samples were proven to have a potential use in photocatalyst, but suffered from photodissolution and memory effect. The mechanism of the photocatalysis was analyzed, and the stability and recycling ability were also evaluated and enhanced.
Keywords: Hierarchical architecture; Nanowire arrays; Photocatalytic activity; Semiconductor.
Figures







References
-
- Wang R, Guo J, Chen D, Miao Y, Pan J, Tjiub W, Liu T. “Tube brush” like ZnO/SiO2 hybrid to construct a flexible membrane with enhanced photocatalytic properties and recycling ability. J Mater Chem. 2011;21:19375–19380. doi: 10.1039/c1jm13979g. - DOI
-
- Kargar A, Sun K, Kim S, Lu D, Jing Y, Liu Z, Pan X, Wang D. Three-dimensional ZnO/Si broom-like nanowire heterostructures as photoelectrochemical anodes for solar energy conversion. Phys Status Solidi A. 2013;210:2561–2568. doi: 10.1002/pssa.201329214. - DOI
-
- Cheng C, Fan H. Branched nanowires: synthesis and energy applications. Nano Today. 2012;7:327–343. doi: 10.1016/j.nantod.2012.06.002. - DOI
-
- Bierman M, Jin S. Potential applications of hierarchical branching nanowires in solar energy conversion. Energy Environ Sci. 2009;2:1050–1059. doi: 10.1039/b912095e. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

