Neuronal apoptosis inhibitory protein (NAIP) localizes to the cytokinetic machinery during cell division
- PMID: 28059125
- PMCID: PMC5216396
- DOI: 10.1038/srep39981
Neuronal apoptosis inhibitory protein (NAIP) localizes to the cytokinetic machinery during cell division
Abstract
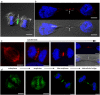
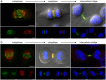
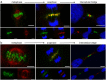
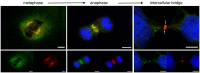
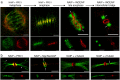
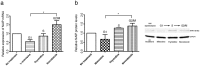
The neuronal apoptosis inhibitory protein (NAIP) is a constituent of the inflammasome and a key component of the innate immune system. Here we use immunofluorescence to position NAIP within the cytokinetic apparatus, contiguous to chromosomal passenger complex (CPC), Centralspindlin, PRC1 and KIF4A. During metaphase, NAIP accumulates in the mitotic spindle poles and is shown in spindle microtubules; in anaphase NAIP is detected in the middle of the central spindle. At the end of cytokinesis, NAIP is localized in the outlying region of the stem body, the center of the intercellular bridge formed between daughter cells prior to cellular abscission. We also describe the sustained presence of NAIP mRNA and protein throughout the cell cycle with a significant increase observed in the G2/M phase. Consistent with a role for NAIP in cytokinesis, NAIP overexpression in HeLa cells promotes the acquisition of a multinuclear phenotype. Conversely, NAIP siRNA gene silencing results in an apoptotic lethal phenotype. Our confocal and super resolution stimulated-emission-depletion (STED) examination of mammalian cell cytokinesis demonstrate a potential new role for NAIP in addition to anti-apoptotic and innate immunology functions.
Figures

References
-
- Fededa J. P. & Gerlich D. W. Molecular control of animal cell cytokinesis. Nature Cell Biology 14, 440–447 (2012). - PubMed
-
- Roy N. et al.. The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell 80, 167–178 (1995). - PubMed
-
- Liston P. et al.. Suppression of apoptosis in mammalian cells by naip and a related family of iap genes. Nature 379, 349–353 (1996). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

