Molecular characterization of pneumococcal surface protein K, a potential pneumococcal vaccine antigen
- PMID: 28059611
- PMCID: PMC5626202
- DOI: 10.1080/21505594.2016.1278334
Molecular characterization of pneumococcal surface protein K, a potential pneumococcal vaccine antigen
Abstract
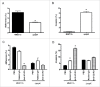
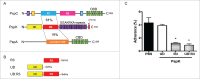
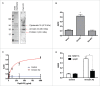
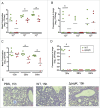
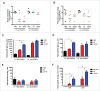
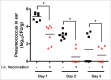
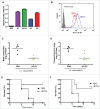
The pneumococcal capsule is indispensable for pathogenesis in systemic infections; however, many pneumococcal diseases, including conjunctivitis, otitis media, and some systemic infections in immunocompromised patients, are caused by nonencapsulated Streptococcus pneumoniae (NESp). Null capsule clade 1 (NCC1), found in group 2 NESp, expresses pneumococcal surface protein K (PspK) and is becoming prevalent among pneumococcal organisms owing to the widespread use of pneumococcal conjugate vaccines. Despite its clinical importance, the molecular mechanisms underlying the prevalence of NCC1 have not been fully elucidated. Here, we investigated the role of the R3 domain of PspK in the epithelial cell adherence of NCC1. We found that the R3 domain of PspK mediated NCC1 adherence via its direct interaction with the epithelial surface protein annexin A2. Additionally, neutralization with purified recombinant PspK-R3 or rabbit anti-UD:R3 IgG inhibited binding of NESp to lung epithelial cells in vitro. Immunization with the 'repeat' domain of PspK-R3 or PspK-UD:R3 effectively elicited mucosal and systemic immune responses against PspK-R3 and provided protection against nasopharyngeal, lung, and middle ear colonization of NESp in mice. Additionally, we found that rabbit anti-UD:R3 IgG bound to PspC-R1 of the encapsulated TIGR4 strain and that UD:R3 immunization provided protection against nasopharyngeal and lung colonization of TIGR4 and deaths by TIGR4 and D39 in mice. Further studies using 68 pneumococcal clinical isolates showed that 79% of clinical isolates showed cross-reactivity to rabbit anti-UD:R3 IgG. About 87% of serotypes in the 13-valent pneumococcal conjugate vaccine (PCV) and 68% of non-vaccine serotypes were positive for cross-reactivity with rabbit anti-UD:R3 IgG. Thus, the R3 domain of PspK may be an effective vaccine candidate for both NESp and encapsulated Sp.
Keywords: Streptococcus pneumoniae; annexin A2; pneumococcal surface protein K; vaccine.
Figures
Comment in
-
Non-encapsulated Streptococcus pneumoniae, vaccination as a measure to interfere with horizontal gene transfer.Virulence. 2017 Aug 18;8(6):637-639. doi: 10.1080/21505594.2017.1309492. Epub 2017 Mar 22. Virulence. 2017. PMID: 28328284 Free PMC article. No abstract available.
References
-
- Zhu F, Hu Y, Li J, Ye Q, Young MM Jr., Zhou X, Chen Z, Yan B, Liang JZ, Gruber WC, et al.. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine compared with 7-valent pneumococcal conjugate vaccine among healthy infants in China. Pediatr Infect Dis J 2016; :999-1010; PMID:27254028; http://dx.doi.org/ 10.1097/INF.0000000000001248 - DOI - PubMed
-
- van Werkhoven CH, Hollingsworth RC, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Sanders EA, Bonten MJ. Pneumococcal conjugate vaccine herd effects on non-invasive pneumococcal pneumonia in elderly. Vaccine 2016; 34(28):3275-82; PMID:27171754; http://dx.doi.org/ 10.1016/j.vaccine.2016.05.002 - DOI - PubMed
-
- Valente C, Hinds J, Gould KA, Pinto FR, de Lencastre H, Sa-Leao R. Impact of the 13-valent pneumococcal conjugate vaccine on Streptococcus pneumoniae multiple serotype carriage. Vaccine 2016; 34(34):4072-8; PMID:27325351; http://dx.doi.org/ 10.1016/j.vaccine.2016.06.017 - DOI - PubMed
-
- Grant LR, Hammitt LL, O'Brien SE, Jacobs MR, Donaldson C, Weatherholtz RC, Reid R, Santosham M, O'Brien KL. Impact of the 13-valent pneumococcal conjugate vaccine on pneumococcal carriage among American Indians. Pediatr Infect Dis J 2016; 35(8):907-14; PMID:27171679; http://dx.doi.org/ 10.1097/INF.0000000000001207 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
