Comparison of iatrogenic pain between rotavirus vaccination before and after vaccine injection in 2-month-old infants
- PMID: 28059619
- PMCID: PMC5443369
- DOI: 10.1080/21645515.2016.1267082
Comparison of iatrogenic pain between rotavirus vaccination before and after vaccine injection in 2-month-old infants
Abstract
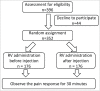
Oral rotavirus vaccine (RV) administration in conjunction with other injectable vaccines has been used worldwide. However, whether the sequence of RV administration is associated with the reduction of injection-induced pain remains unclear. In this randomized controlled trial, we enrolled 6-12-wk-old healthy infants. The pain response of the infants was scored on the basis of their crying, irritability, facial expression, gagging and distress. A multivariate logistic regression model was used to compare the pain response after adjustment for possible confounders. We enrolled 352 infants, of whom 176 infants received RV before injection (experimental group) and 176 infants received an RV after injection (comparison group). Sex, number of injections, main caregiver, feeding type, and RV type did not differ significantly between the 2 groups. Multivariate regression analyses showed that, at 30 s after the intervention, the episode of gagging was more frequent in the comparison group than in the experimental group (p = 0.004). At 180 s after the intervention, the infants cried more often in the comparison group (p < 0.001). Furthermore, the infants in the experimental group more often relaxed (p < 0.001), rested quietly (p = 0.001), and were smiling (p = 0.001) than did those in the comparison group. Our results indicate that compared with oral RV administration after injection, oral RV administration before injection is more effective in reducing injection-induced pain in 2-mo-old infants. The findings can provide a clinical strategy for relieving pain from vaccination in young infants.
Keywords: infant; injection; pain; rotavirus vaccine; vaccination.
Figures
References
-
- Cherian T, Wang S, Mantel C. Rotavirus vaccines in developing countries: the potential impact, implementation challenges, and remaining questions. Vaccine 2012; 30 (Suppl 1):A3-6; PMID:22520133; http://dx.doi.org/10.1016/j.vaccine.2011.10.007 - DOI - PubMed
-
- Yen C, Tate JE, Patel MM, Cortese MM, Lopman B, Fleming J, Lewis K, Jiang B, Gentsch JR, Steele AD, et al.. Rotavirus vaccines: Update on global impact and future priorities. Human Vaccines 2011; 7(12):1282-90; PMID:22108032; http://dx.doi.org/10.4161/hv.7.12.18321 - DOI - PMC - PubMed
-
- Marshall GS, Adams GL, Leonardi ML, Petrecz M, Flores SA, Ngai AL, Xu J, Liu G, Stek JE, Foglia G, et al.. Immunogenicity, safety, and tolerability of a hexavalent vaccine in infants. Pediatrics 2015; 136(2):e323-32; PMID:26216331; http://dx.doi.org/10.1542/peds.2014-4102 - DOI - PubMed
-
- Glass RI, Parashar UD, Bresee JS, Turcios R, Fischer TK, Widdowson MA, Jiang B, Gentsch JR. Rotavirus vaccines: current prospects and future challenges. Lancet 2006; 368(9532):323-32; PMID:16860702; http://dx.doi.org/10.1016/S0140-6736(06)68815-6 - DOI - PubMed
-
- Thrane SE, Wanless S, Cohen SM, Danford CA. The assessment and non-pharmacologic treatment of procedural pain from infancy to school age through a developmental lens: A synthesis of evidence with recommendations. J Pediatr Nurs 2016; 31(1):e23-32; PMID:26424196; http://dx.doi.org/10.1016/j.pedn.2015.09.002 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical