Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation
- PMID: 28059703
- PMCID: PMC5257256
- DOI: 10.7554/eLife.21330
Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation
Abstract
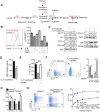
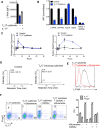
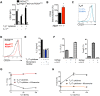
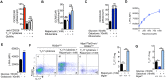
Rapidly proliferating cells switch from oxidative phosphorylation to aerobic glycolysis plus glutaminolysis, markedly increasing glucose and glutamine catabolism. Although Otto Warburg first described aerobic glycolysis in cancer cells >90 years ago, the primary purpose of this metabolic switch remains controversial. The hexosamine biosynthetic pathway requires glucose and glutamine for de novo synthesis of UDP-GlcNAc, a sugar-nucleotide that inhibits receptor endocytosis and signaling by promoting N-acetylglucosamine branching of Asn (N)-linked glycans. Here, we report that aerobic glycolysis and glutaminolysis co-operatively reduce UDP-GlcNAc biosynthesis and N-glycan branching in mouse T cell blasts by starving the hexosamine pathway of glucose and glutamine. This drives growth and pro-inflammatory TH17 over anti-inflammatory-induced T regulatory (iTreg) differentiation, the latter by promoting endocytic loss of IL-2 receptor-α (CD25). Thus, a primary function of aerobic glycolysis and glutaminolysis is to co-operatively limit metabolite supply to N-glycan biosynthesis, an activity with widespread implications for autoimmunity and cancer.
Keywords: N-acetylglucosamine; N-glycosylation; T cell; Warburg effect; cell biology; glutaminolysis; glycolysis; immunology; mouse.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures



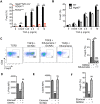


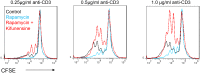
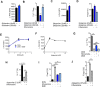
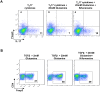
References
-
- Chang CH, Curtis JD, Maggi LB, Faubert B, Villarino AV, O'Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, Weber JD, Pearce EJ, Jones RG, Pearce EL. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. - DOI - PMC - PubMed
-
- Cummings RD, Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. The Journal of Biological Chemistry. 1982;257:11230–11234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

