DPPIV promotes endometrial carcinoma cell proliferation, invasion and tumorigenesis
- PMID: 28060721
- PMCID: PMC5352432
- DOI: 10.18632/oncotarget.14412
DPPIV promotes endometrial carcinoma cell proliferation, invasion and tumorigenesis
Abstract
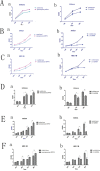
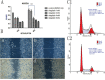
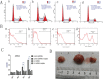
Dipeptidyl peptidase IV (DPPIV), also known as CD26, is a 110-kDa cell surface glycoprotein expressed in various tissues. DPPIV reportedly plays a direct role in the progression of several human malignancies. DPPIV specific inhibitors are employed as antidiabetics and could potentially be repurposed to enhance anti-tumor immunotherapies. In the present study, we investigated the correlation between DPPIV expression and tumor progression in endometrial carcinoma (EC). DPPIV overexpression altered cell morphology and stimulated cell proliferation, invasion and tumorigenesis in vitro and in vivo. These effects were abrogated by DPPIV knockdown or pharmacological inhibition using sitagliptin. DPPIV overexpression increased hypoxia-inducible factor 1a (HIF-1a) and vascular endothelial growth factor A (VEGFA) expression to promote HIF-1a-VEGFA signaling. Our results indicated that DPPIV accelerated endometrial carcinoma progression and that sitagliptin may be an effective anti-EC therapeutic.
Keywords: dipeptidyl peptidase IV; endometrial carcinoma; hypoxia-inducible factor 1a; sitagliptin; vascular endothelial growth factor A.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Harkenrider MM, Block AM, Siddiqui ZA, Small W., Jr The role of vaginal cuff brachytherapy in endometrial cancer. Gynecologic oncology. 2015;136:365–372. - PubMed
-
- Jin F, Devesa SS, Zheng W, Blot WJ, Fraumeni JF, Jr, Gao YT. Cancer incidence trends in urban Shanghai, 1972-1989. International journal of cancer. 1993;53:764–770. - PubMed
-
- Fader AN, Arriba LN, Frasure HE, von Gruenigen VE. Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship. Gynecologic oncology. 2009;114:121–127. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

