Estimating the Cost-Effectiveness of One-Time Screening and Treatment for Hepatitis C in Korea
- PMID: 28060834
- PMCID: PMC5218507
- DOI: 10.1371/journal.pone.0167770
Estimating the Cost-Effectiveness of One-Time Screening and Treatment for Hepatitis C in Korea
Abstract
Background and aims: This study aims to investigate the cost-effectiveness of a one-time hepatitis C virus (HCV) screening and treatment program in South Korea where hepatitis B virus (HBV) prevails, in people aged 40-70, compared to current practice (no screening).
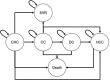
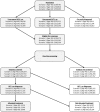
Methods: A published Markov model was used in conjunction with a screening and treatment decision tree to model patient cohorts, aged 40-49, 50-59 and 60-69 years, distributed across chronic hepatitis C (CHC) and compensated cirrhosis (CC) health states (82.5% and 17.5%, respectively). Based on a published seroepidemiology study, HCV prevalence was estimated at 0.60%, 0.80% and 1.53%, respectively. An estimated 71.7% of the population was screened. Post-diagnosis, 39.4% of patients were treated with a newly available all-oral direct-acting antiviral (DAA) regimen over 5 years. Published rates of sustained virologic response, disease management costs, transition rates and utilities were utilised.
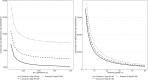
Results: Screening resulted in the identification of 43,635 previously undiagnosed patients across all cohorts. One-time HCV screening and treatment was estimated to be cost-effective across all cohorts; predicted incremental cost-effectiveness ratios ranged from $5,714 to $8,889 per quality-adjusted life year gained. Incremental costs associated with screening, treatment and disease management ranged from $156.47 to $181.85 million USD; lifetime costs-offsets associated with the avoidance of end stage liver disease complications ranged from $51.47 to $57.48 million USD.
Conclusions: One-time HCV screening and treatment in South Korean people aged 40-70 is likely to be highly cost-effective compared to the current practice of no screening.
Conflict of interest statement
The authors declare this commercial funder, Bristol-Myers Squibb Pharmaceuticals Ltd., along with any other relevant declarations relating to employment, consultancy, patents, products in development, marketed products, etc. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- Sievert W, Altraif I, Razavi H, Abdo A, Ahmed EA, Alomair A, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int 2011;31(Suppl 2):61–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

