Genomes of Fasciola hepatica from the Americas Reveal Colonization with Neorickettsia Endobacteria Related to the Agents of Potomac Horse and Human Sennetsu Fevers
- PMID: 28060841
- PMCID: PMC5257007
- DOI: 10.1371/journal.pgen.1006537
Genomes of Fasciola hepatica from the Americas Reveal Colonization with Neorickettsia Endobacteria Related to the Agents of Potomac Horse and Human Sennetsu Fevers
Abstract
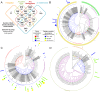
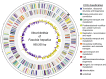
Food borne trematodes (FBTs) are an assemblage of platyhelminth parasites transmitted through the food chain, four of which are recognized as neglected tropical diseases (NTDs). Fascioliasis stands out among the other NTDs due to its broad and significant impact on both human and animal health, as Fasciola sp., are also considered major pathogens of domesticated ruminants. Here we present a reference genome sequence of the common liver fluke, Fasciola hepatica isolated from sheep, complementing previously reported isolate from cattle. A total of 14,642 genes were predicted from the 1.14 GB genome of the liver fluke. Comparative genomics indicated that F. hepatica Oregon and related food-borne trematodes are metabolically less constrained than schistosomes and cestodes, taking advantage of the richer millieux offered by the hepatobiliary organs. Protease families differentially expanded between diverse trematodes may facilitate migration and survival within the heterogeneous environments and niches within the mammalian host. Surprisingly, the sequencing of Oregon and Uruguay F. hepatica isolates led to the first discovery of an endobacteria in this species. Two contigs from the F. hepatica Oregon assembly were joined to complete the 859,205 bp genome of a novel Neorickettsia endobacterium (nFh) closely related to the etiological agents of human Sennetsu and Potomac horse fevers. Immunohistochemical studies targeting a Neorickettsia surface protein found nFh in specific organs and tissues of the adult trematode including the female reproductive tract, eggs, the Mehlis' gland, seminal vesicle, and oral suckers, suggesting putative routes for fluke-to-fluke and fluke-to-host transmission. The genomes of F. hepatica and nFh will serve as a resource for further exploration of the biology of F. hepatica, and specifically its newly discovered trans-kingdom interaction with nFh and the impact of both species on disease in ruminants and humans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, et al. World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis. PLoS Med. 2015;12(12):e1001920 10.1371/journal.pmed.1001920 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

