Impact of vessel wall lesions and vascular stenoses on cerebrovascular reactivity in patients with intracranial stenotic disease
- PMID: 28061015
- PMCID: PMC5500451
- DOI: 10.1002/jmri.25602
Impact of vessel wall lesions and vascular stenoses on cerebrovascular reactivity in patients with intracranial stenotic disease
Abstract
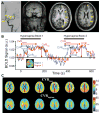
Purpose: To compare cerebrovascular reactivity (CVR) and CVR lagtimes in flow territories perfused by vessels with vs. without proximal arterial wall disease and/or stenosis, separately in patients with atherosclerotic and nonatherosclerotic (moyamoya) intracranial stenosis.
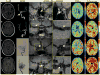
Materials and methods: Atherosclerotic and moyamoya patients with >50% intracranial stenosis and <70% cervical stenosis underwent angiography, vessel wall imaging (VWI), and CVR-weighted imaging (n = 36; vessel segments evaluated = 396). Angiography and VWI were evaluated for stenosis locations and vessel wall lesions. Maximum CVR and CVR lagtime were contrasted between vascular territories with and without proximal intracranial vessel wall lesions and stenosis, and a Wilcoxon rank-sum was test used to determine differences (criteria: corrected two-sided P < 0.05).
Results: CVR lagtime was prolonged in territories with vs. without a proximal vessel wall lesion or stenosis for both patient groups: moyamoya (CVR lagtime = 45.5 sec ± 14.2 sec vs. 35.7 sec ± 9.7 sec, P < 0.001) and atherosclerosis (CVR lagtime = 38.2 sec ± 9.1 sec vs. 35.0 sec ± 7.2 sec, P = 0.001). For reactivity, a significant decrease in maximum CVR in the moyamoya group only (maximum CVR = 9.8 ± 2.2 vs. 12.0 ± 2.4, P < 0.001) was observed.
Conclusion: Arterial vessel wall lesions detected on noninvasive, noncontrast intracranial VWI in patients with intracranial stenosis correlate on average with tissue-level impairment on CVR-weighted imaging.
Level of evidence: 4 Technical Efficacy: Stage 3 J. Magn. Reson. Imaging 2017;46:1167-1176.
Keywords: cerebrovascular reactivity; intracranial stenosis; moyamoya; vessel wall imaging.
© 2017 International Society for Magnetic Resonance in Medicine.
Figures

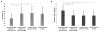

References
-
- Ovbiagele B, Cruz-Flores S, Lynn MJ, Chimowitz MI. Early stroke risk after transient ischemic attack among individuals with symptomatic intracranial artery stenosis. Arch Neurol. 2008;65(6):733–737. - PubMed
-
- Zaidat OO, Fitzsimmons BF, Woodward BK, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. Jama. 2015;313(12):1240–1248. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

