Oxidative stress biomarkers and their relationship with cytokine concentrations in overweight/obese pregnant women and their neonates
- PMID: 28061809
- PMCID: PMC5219784
- DOI: 10.1186/s12865-016-0184-6
Oxidative stress biomarkers and their relationship with cytokine concentrations in overweight/obese pregnant women and their neonates
Abstract
Background: Oxidative damage present in obese/overweight mothers may lead to further oxidative stress conditions or inflammation in maternal and cord blood samples. Thirty-four pregnant women/newborn pairs were included in this study to assess the presence of oxidative stress biomarkers and their relationship with serum cytokine concentrations. Oxidative stress biomarkers and antioxidant enzymes were compared between the mother/offspring pairs. The presence of 27 cytokines was measured in maternal and cord blood samples. Analyses were initially performed between all mothers and newborns and later between normal weight and mothers with overweight and obesity, and diabetic/non-diabetic women.
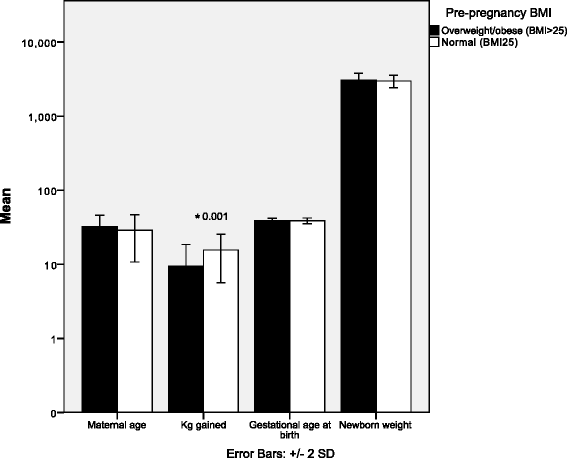
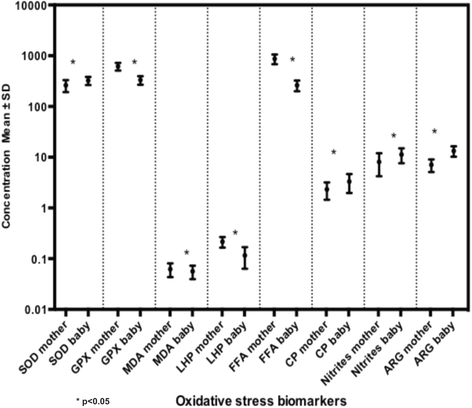
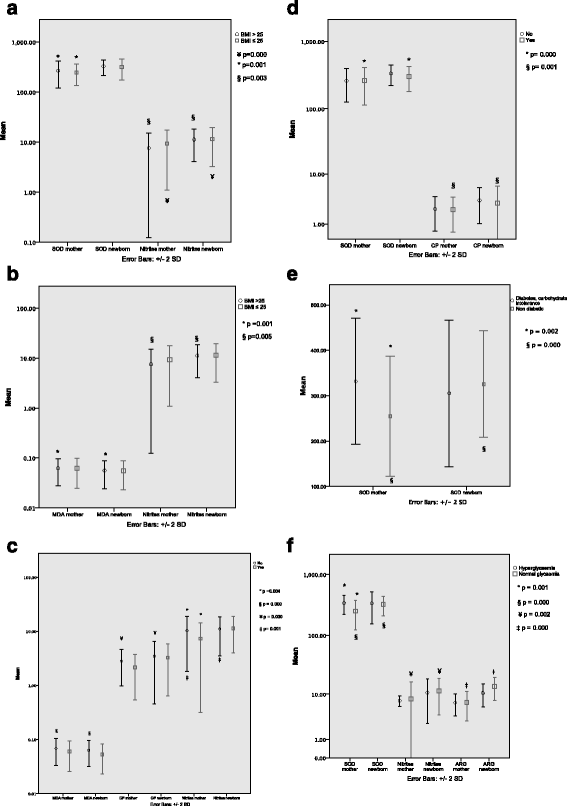
Results: Significant differences were found in biomarker concentrations between mothers and newborns. Additionally, superoxide-dismutase activity was higher in pre-pregnancy overweight mothers compared to those with normal weight. Activity for this enzyme was higher in neonates born from mothers with normal pregestational weight compared with their mothers. Nitrites in overweight/obese mothers were statistically lower than in their offspring. Maternal free fatty acids, nitrites, carbonylated proteins, malondialdehyde and superoxide dismutase predicted maternal serum concentrations of IL-4, IL-13, IP-10 and MIP-1β. Arginase activity in maternal plasma was related to decreased concentrations of IL-4 and IL-1β in cord arterial blood. Increased maternal malondialdehyde plasma was associated with higher levels of IL-6 and IL-7 in the offspring.
Conclusions: Oxidative stress biomarkers differ between mothers and offspring and can predict maternal and newborn cytokine concentrations, indicating a potential role for oxidative stress in foetal metabolic and immunologic programming. Moreover, maternal obesity and diabetes may affect maternal microenvironments, and oxidative stress related to these can have an impact on the placenta and foetal growth.
Keywords: Arginase; Cytokines; Diabetes; Free fatty acids; Nitrites; Obesity; Offspring; Oxidative stress; Pregnancy; Vitamin supplementation.
Figures
References
-
- Heslehurst N, Simpson H, Ells LJ, Rankin J, Wilkinson J, Lang R, Brown TJ, Summerbell CD. The impact of maternal bmi status on pregnancy outcomes with immediate short-term obstetric resource implications: A meta-analysis. Obes Rev. 2008;9(6):635–683. doi: 10.1111/j.1467-789X.2008.00511.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

