Age Nutrition Chirugie (ANC) study: impact of a geriatric intervention on the screening and management of undernutrition in elderly patients operated on for colon cancer, a stepped wedge controlled trial
- PMID: 28061830
- PMCID: PMC5219771
- DOI: 10.1186/s12877-016-0402-3
Age Nutrition Chirugie (ANC) study: impact of a geriatric intervention on the screening and management of undernutrition in elderly patients operated on for colon cancer, a stepped wedge controlled trial
Abstract
Background: Undernutrition prior to major abdominal surgery is frequent and increases morbidity and mortality, especially in older patients. The management of undernutrition reduces postoperative complications. Nutritional management should be a priority in patient care during the preoperative period. However undernutrition is rarely detected and the guidelines are infrequently followed. Preoperative undernutrition screening should allow a better implementation of the guidelines.
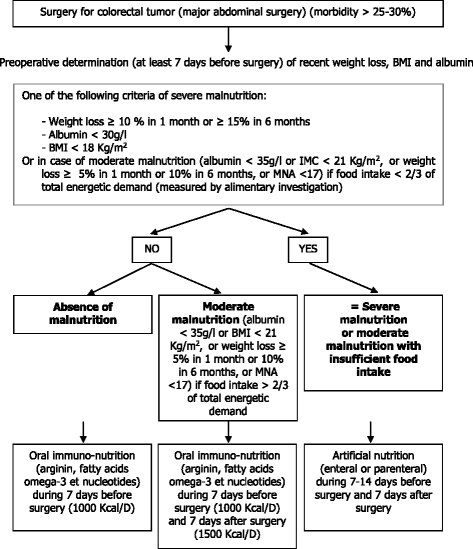
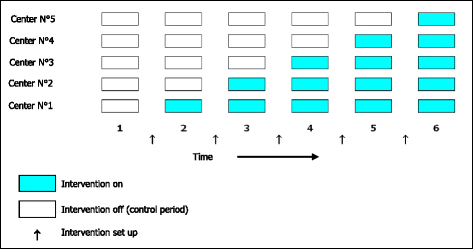
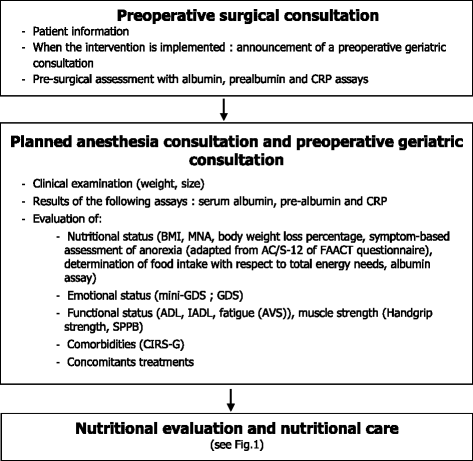
Methods/design: The ANC ("Age Nutrition Chirurgie") study is an interventional, comparative, prospective, multicenter, randomized protocol based on the stepped wedge trial design. For the intervention, the surgeon will inform the patient of the establishment of a systematic preoperative geriatric assessment that will allow the preoperative diagnosis of the nutritional status and the implementation of an adjusted nutritional support in accordance with the nutritional guidelines. The primary outcome measure is to determine the impact of the geriatric intervention on the level of perioperative nutritional management, in accordance with the current European guidelines. The implementation of the intervention in the five participating centers will be rolled-out sequentially over six time periods (every six months). Investigators must recommend that all patients aged 70 years or over and who are consulting for a surgery for a colorectal cancer should consider participating in this study.
Discussion: The ANC study is based on an original methodology, the stepped wedge trial design, which is appropriate for evaluating the implementation of a geriatric and nutritional assessment during the perioperative period. We describe the purpose of this geriatric intervention, which is expected to apply the ESPEN and SFNEP recommendations through the establishment of an undernutrition screening and a management program for patients with cancer. This intervention should allow a decrease in patient morbidity and mortality due to undernutrition.
Trial registration: This study is registered in ClinicalTrials.gov NCT02084524 on March 11, 2014 (retrospectively registered).
Keywords: Cancer; Colorectal tumor; Nutrition; Stepped wedge; Vulnerable.
Figures

References
-
- HAS . Stratégie de prise en charge en cas de dénutrition protéino-énergétique chez la personne âgée. 2007.
-
- Bélot A, Velten M, Grosclaude P, et al. In: Estimation nationale de l’incidence et de la mortalité par cancer en France entre 1980 et 2005. Saint-Maurice (Fra)et al., editors. Luxembourg: Institut National de Veille Sanitaire; 2008.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

