A randomized, seven-day study to assess the efficacy and safety of a glycopyrrolate/formoterol fumarate fixed-dose combination metered dose inhaler using novel Co-Suspension™ Delivery Technology in patients with moderate-to-very severe chronic obstructive pulmonary disease
- PMID: 28061907
- PMCID: PMC5216561
- DOI: 10.1186/s12931-016-0491-8
A randomized, seven-day study to assess the efficacy and safety of a glycopyrrolate/formoterol fumarate fixed-dose combination metered dose inhaler using novel Co-Suspension™ Delivery Technology in patients with moderate-to-very severe chronic obstructive pulmonary disease
Erratum in
-
Erratum to: A randomized, seven-day study to assess the efficacy and safety of a glycopyrrolate/formoterol fumarate fixed-dose combination metered dose inhaler using novel Co-Suspension™ Delivery Technology in patients with moderate-to-very severe chronic obstructive pulmonary disease.Respir Res. 2017 Aug 21;18(1):158. doi: 10.1186/s12931-017-0638-2. Respir Res. 2017. PMID: 28826397 Free PMC article. No abstract available.
Abstract
Background: Long-acting muscarinic antagonist/long-acting β2-agonist combinations are recommended for patients whose chronic obstructive pulmonary disease (COPD) is not managed with monotherapy. We assessed the efficacy and safety of glycopyrrolate (GP)/formoterol fumarate (FF) fixed-dose combination delivered via a Co-Suspension™ Delivery Technology-based metered dose inhaler (MDI) (GFF MDI).
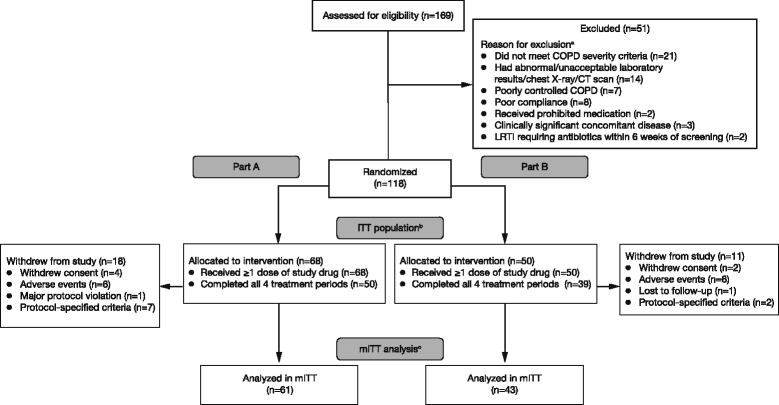
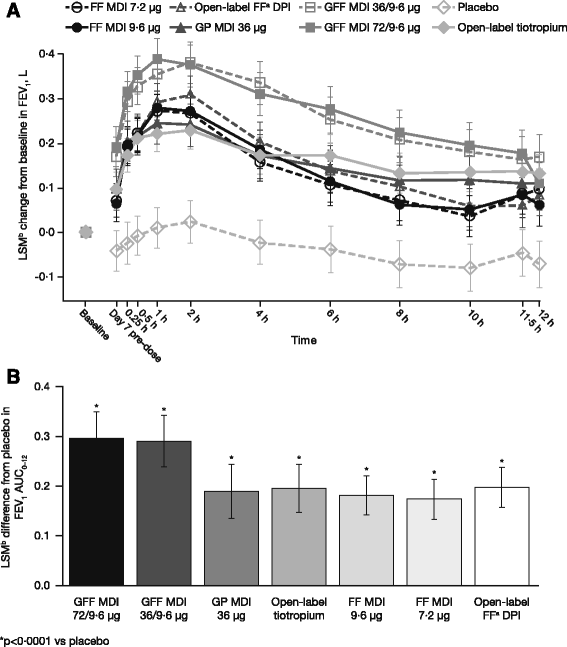
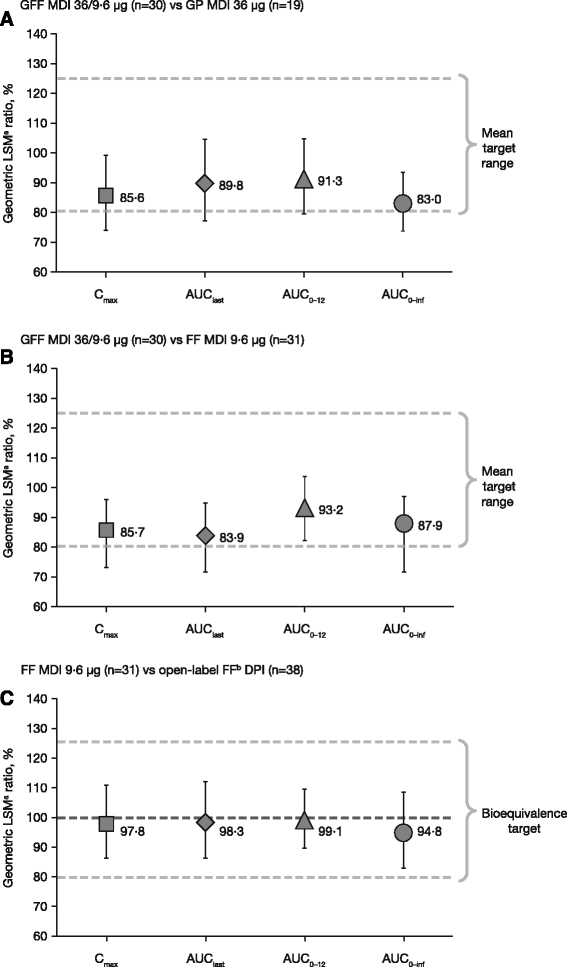
Methods: This was a Phase IIb randomized, multicenter, placebo-controlled, double-blind, chronic-dosing (7 days), crossover study in patients with moderate-to-very severe COPD ( NCT01085045 ). Treatments included GFF MDI twice daily (BID) (GP/FF 72/9.6 μg or 36/9.6 μg), GP MDI 36 μg BID, FF MDI 7.2 and 9.6 μg BID, placebo MDI, and open-label formoterol dry powder inhaler (FF DPI) 12 μg BID or tiotropium DPI 18 μg once daily. The primary endpoint was forced expiratory volume in 1 s area under the curve from 0 to 12 h (FEV1 AUC0-12) on Day 7 relative to baseline FEV1. Secondary endpoints included pharmacokinetics and safety.
Results: GFF MDI 72/9.6 μg or 36/9.6 μg led to statistically significant improvements in FEV1 AUC0-12 after 7 days' treatment versus monocomponent MDIs, placebo MDI, tiotropium, or FF DPI (p ≤ 0.0002). GFF MDI 36/9.6 μg was non-inferior to GFF MDI 72/9.6 μg and monocomponent MDIs were non-inferior to open-label comparators. Pharmacokinetic results showed glycopyrrolate and formoterol exposure were decreased following administration via fixed-dose combination versus monocomponent MDIs; however, this was not clinically meaningful. GFF MDI was well tolerated.
Conclusions: GFF MDI 72/9.6 μg and 36/9.6 μg BID improve lung function and are well tolerated in patients with moderate-to-very severe COPD.
Trial registration: ClinicalTrials.gov NCT01085045 . Registered 9 March 2010.
Keywords: Bronchodilators; COPD; COPD maintenance; Co-Suspension™ Delivery Technology; LABA; LAMA; Lung function; Metered dose inhaler.
Figures
References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Diseases (GOLD) 2016 2016. [http://www.goldcopd.org]. Accessed 16 June 2016.
-
- Yu AP, Guerin A, Ponce de Leon D, Ramakrishnan K, Wu EQ, Mocarski M, et al. Therapy persistence and adherence in patients with chronic obstructive pulmonary disease: multiple versus single long-acting maintenance inhalers. J Med Econ. 2011;14:486–496. doi: 10.3111/13696998.2011.594123. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

