Time to Change Dosing of Inactivated Quadrivalent Influenza Vaccine in Young Children: Evidence From a Phase III, Randomized, Controlled Trial
- PMID: 28062552
- PMCID: PMC5907868
- DOI: 10.1093/jpids/piw068
Time to Change Dosing of Inactivated Quadrivalent Influenza Vaccine in Young Children: Evidence From a Phase III, Randomized, Controlled Trial
Abstract
Background.: Children under 3 years of age may benefit from a double-dose of inactivated quadrivalent influenza vaccine (IIV4) instead of the standard-dose.
Methods.: We compared the only United States-licensed standard-dose IIV4 (0.25 mL, 7.5 µg hemagglutinin per influenza strain) versus double-dose IIV4 manufactured by a different process (0.5 mL, 15 µg per strain) in a phase III, randomized, observer-blind trial in children 6-35 months of age (NCT02242643). The primary objective was to demonstrate immunogenic noninferiority of the double-dose for all vaccine strains 28 days after last vaccination. Immunogenic superiority of the double-dose was evaluated post hoc. Immunogenicity was assessed in the per-protocol cohort (N = 2041), and safety was assessed in the intent-to-treat cohort (N = 2424).
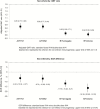
Results.: Immunogenic noninferiority of double-dose versus standard-dose IIV4 was demonstrated in terms of geometric mean titer (GMT) ratio and seroconversion rate difference. Superior immunogenicity against both vaccine B strains was observed with double-dose IIV4 in children 6-17 months of age (GMT ratio = 1.89, 95% confidence interval [CI] = 1.64-2.17, B/Yamagata; GMT ratio = 2.13, 95% CI = 1.82-2.50, B/Victoria) and in unprimed children of any age (GMT ratio = 1.85, 95% CI = 1.59-2.13, B/Yamagata; GMT ratio = 2.04, 95% CI = 1.79-2.33, B/Victoria). Safety and reactogenicity, including fever, were similar despite the higher antigen content and volume of the double-dose IIV4. There were no attributable serious adverse events.
Conclusions.: Double-dose IIV4 may improve protection against influenza B in some young children and simplifies annual influenza vaccination by allowing the same vaccine dose to be used for all eligible children and adults.
Keywords: children; double-dose; inactivated quadrivalent influenza vaccine..
© The Author 2017. Published by Oxford University Press on behalf of The Journal of the Pediatric Infectious Diseases Society
Figures



References
-
- O’Brien MA, Uyeki TM, Shay DK, et al. Incidence of outpatient visits and hospitalizations related to influenza in infants and young children. Pediatrics 2004; 113:585–93. - PubMed
-
- Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med 2000; 342:232–9. - PubMed
-
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086–96. - PubMed
-
- Bourgeois FT, Valim C, Wei JC, et al. Influenza and other respiratory virus-related emergency department visits among young children. Pediatrics 2006; 118:e1–8. - PubMed
-
- Centers for Disease Control and Prevention (CDC). Influenza-associated pediatric deaths–United States, September 2010-August 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1233–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

