Fosfomycin: Mechanism and Resistance
- PMID: 28062557
- PMCID: PMC5287057
- DOI: 10.1101/cshperspect.a025262
Fosfomycin: Mechanism and Resistance
Abstract
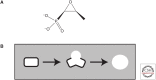
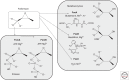
Fosfomycin, a natural product antibiotic, has been in use for >20 years in Spain, Germany, France, Japan, Brazil, and South Africa for urinary tract infections (UTIs) and other indications and was registered in the United States for the oral treatment of uncomplicated UTIs because of Enterococcus faecalis and Escherichia coli in 1996. It has a broad spectrum, is bactericidal, has very low toxicity, and acts as a time-dependent inhibitor of the MurA enzyme, which catalyzes the first committed step of peptidoglycan synthesis. Whereas resistance to fosfomycin arises rapidly in vitro through loss of active transport mechanisms, resistance is rarely seen during therapy of UTIs, seemingly because of the low fitness of the resistant organisms. Recently, interest has grown in the use of fosfomycin against multidrug-resistant (MDR) pathogens in other indications, prompting the advent of development in the United States of a parenteral formulation for use, initially, in complicated UTIs. Whereas resistance has not been problematic in the uncomplicated UTI setting, it remains to be seen whether resistance remains at bay with expansion to other indications.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
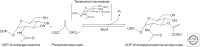
References
-
- Blake KL, O’Neill AJ, Mengin-Lecreulx D, Henderson PJ, Bostock JM, Dunsmore CJ, Simmons KJ, Fishwick CW, Leeds JA, Chopra I. 2009. The nature of Staphylococcus aureus MurA and MurZ and approaches for detection of peptidoglycan biosynthesis inhibitors. Mol Microbiol 72: 335–343. - PubMed
-
- Brown ED, Marquardt JL, Lee JP, Walsh CT, Anderson KS. 1994. Detection and characterization of a phospholactoyl-enzyme adduct in the reaction catalyzed by UDP-N-acetylglucosamine enolpyruvoyl transferase, MurZ. Biochemistry 33: 10638–10645. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
