Cardiolipin Regulates Mitophagy through the Protein Kinase C Pathway
- PMID: 28062576
- PMCID: PMC5314186
- DOI: 10.1074/jbc.M116.753574
Cardiolipin Regulates Mitophagy through the Protein Kinase C Pathway
Abstract
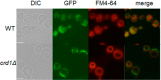
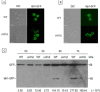
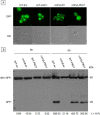
Cardiolipin (CL), the signature phospholipid of mitochondrial membranes, is important for cardiovascular health, and perturbation of CL metabolism is implicated in cardiovascular disease. Although the role of CL in mitochondrial function, biogenesis, and genome stability has been studied, recent findings indicate that it is essential for functions apart from mitochondrial bioenergetics. In this study, we report that mitophagy is perturbed in CL-deficient yeast cells. Mutants of autophagy/mitophagy genes ATG8, ATG18, and ATG32 synthetically interact with CL synthase mutant crd1Δ. CL-deficient cells exhibited decreased GFP-tagged mitochondrial proteins inside the vacuole and decreased free GFP, consistent with decreased mitophagy. Both PKC and high osmolarity glycerol (HOG) MAPK pathways were shown previously to be required for mitophagy. Activation of both MAPKs was defective in CL-deficient cells. Deletion of HOG pathway genes SHO1, SSK1, STE50, and HOG1 exacerbated crd1Δ growth. 1 m sorbitol and 0.2 m NaCl, which induce the HOG pathway, rescued growth of the mutant. Activation of the MAPK Slt2p was defective in crd1Δ cells, and up-regulation of the PKC pathway by expression of the PKC1R398P gene, which encodes constitutively activated Pkc1p, rescued crd1Δ growth and mitophagy defects. These findings indicate that loss of CL impairs MAPK pathway activation, and decreased activation of the PKC pathway leads to defective mitophagy.
Keywords: cardiolipin; mitochondria; mitogen-activated protein kinase (MAPK); mitophagy; protein kinase C (PKC).
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
-
- Barth P. G., Scholte H. R., Berden J. A., Van der Klei-Van Moorsel J. M., Luyt-Houwen I. E., Van 't Veer-Korthof E. T., Van der Harten J. J., and Sobotka-Plojhar M. A. (1983) An X-linked mitochondrial disease affecting cardiac muscle, skeletal muscle and neutrophil leucocytes. J. Neurol. Sci. 62, 327–355 - PubMed
-
- Bione S., D'Adamo P., Maestrini E., Gedeon A. K., Bolhuis P. A., and Toniolo D. (1996) A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nat. Genet. 12, 385–389 - PubMed
-
- Valianpour F., Wanders R. J. A., Overmars H., Vreken P., Van Gennip A. H., Baas F., Plecko B., Santer R., Becker K., and Barth P. G. (2002) Cardiolipin deficiency in X-linked cardioskeletal myopathy and neutropenia (Barth syndrome, mim 302060): a study in cultured skin fibroblasts. J. Pediatrics 141, 729–733 - PubMed
-
- Schlame M., Towbin J. A., Heerdt P. M., Jehle R., DiMauro S., and Blanck T. J. J. (2002) Deficiency of tetralinoleoyl-cardiolipin in Barth syndrome. Ann. Neurol. 51, 634–637 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

