Glioblastoma on a microfluidic chip: Generating pseudopalisades and enhancing aggressiveness through blood vessel obstruction events
- PMID: 28062831
- PMCID: PMC5464359
- DOI: 10.1093/neuonc/now230
Glioblastoma on a microfluidic chip: Generating pseudopalisades and enhancing aggressiveness through blood vessel obstruction events
Abstract
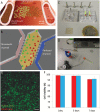
Background: Glioblastoma (GBM) is one of the most lethal tumor types. Hypercellular regions, named pseudopalisades, are characteristic in these tumors and have been hypothesized to be waves of migrating glioblastoma cells. These "waves" of cells are thought to be induced by oxygen and nutrient depletion caused by tumor-induced blood vessel occlusion. Although the universal presence of these structures in GBM tumors suggests that they may play an instrumental role in GBM's spread and invasion, the recreation of these structures in vitro has remained challenging.
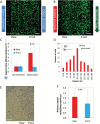
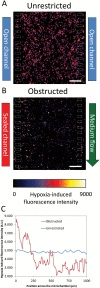
Methods: Here we present a new microfluidic model of GBM that mimics the dynamics of pseudopalisade formation. To do this, we embedded U-251 MG cells within a collagen hydrogel in a custom-designed microfluidic device. By controlling the medium flow through lateral microchannels, we can mimic and control blood-vessel obstruction events associated with this disease.
Results: Through the use of this new system, we show that nutrient and oxygen starvation triggers a strong migratory process leading to pseudopalisade generation in vitro. These results validate the hypothesis of pseudopalisade formation and show an excellent agreement with a systems-biology model based on a hypoxia-driven phenomenon.
Conclusions: This paper shows the potential of microfluidic devices as advanced artificial systems capable of modeling in vivo nutrient and oxygen gradients during tumor evolution.
Keywords: SU-8; glioblastoma; microfluidics; migration; pseudopalisades.
© The Author(s) 2017. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com
Figures



References
-
- Brat DJ, Kaur B, Van Meir EG. Genetic modulation of hypoxia induced gene expression and angiogenesis: relevance to brain tumors. Front Biosci. 2003;8:100–116. - PubMed
-
- Rong Y, Durden DL, Van Meir EG, Brat DJ. ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol. 2006;65(6):529–539. - PubMed
-
- Brat DJ, Castellano-Sanchez AA, Hunter SB, et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64(3):920–927. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

