SF3b1 mutations associated with myelodysplastic syndromes alter the fidelity of branchsite selection in yeast
- PMID: 28062854
- PMCID: PMC5416834
- DOI: 10.1093/nar/gkw1349
SF3b1 mutations associated with myelodysplastic syndromes alter the fidelity of branchsite selection in yeast
Abstract
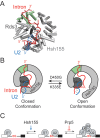
RNA and protein components of the spliceosome work together to identify the 5΄ splice site, the 3΄ splice site, and the branchsite (BS) of nascent pre-mRNA. SF3b1 plays a key role in recruiting the U2 snRNP to the BS. Mutations in human SF3b1 have been linked to many diseases such as myelodysplasia (MDS) and cancer. We have used SF3b1 mutations associated with MDS to interrogate the role of the yeast ortholog, Hsh155, in BS selection and splicing. These alleles change how the spliceosome recognizes the BS and alter splicing when nonconsensus nucleotides are present at the -2, -1 and +1 positions relative to the branchpoint adenosine. This indicates that changes in BS usage observed in humans with SF3b1 mutations may result from perturbation of a conserved mechanism of BS recognition. Notably, different HSH155 alleles elicit disparate effects on splicing: some increase the fidelity of BS selection while others decrease fidelity. Our data support a model wherein conformational changes in SF3b1 promote U2 association with the BS independently of the action of the DEAD-box ATPase Prp5. We propose that SF3b1 functions to stabilize weak U2/BS duplexes to drive spliceosome assembly and splicing.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Coovert D.D., Le T.T., McAndrew P.E., Strasswimmer J., Crawford T.O., Mendell J.R., Coulson S.E., Androphy E.J., Prior T.W., Burghes A.H.. The survival motor neuron protein in spinal muscular atrophy. Hum. Mol. Genet. 1997; 6:1205–1214. - PubMed
-
- McKie A.B., McHale J.C., Keen T.J., Tarttelin E.E., Goliath R., van Lith-Verhoeven J.J., Greenberg J., Ramesar R.S., Hoyng C.B., Cremers F.P. et al. Mutations in the pre-mRNA splicing factor gene PRPC8 in autosomal dominant retinitis pigmentosa (RP13). Hum. Mol. Genet. 2001; 10:1555–1562. - PubMed
-
- Vithana E.N., Abu-Safieh L., Allen M.J., Carey A., Papaioannou M., Chakarova C., Al-Maghtheh M., Ebenezer N.D., Willis C., Moore A.T. et al. A human homolog of yeast pre-mRNA splicing gene, PRP31, underlies autosomal dominant retinitis pigmentosa on chromosome 19q13.4 (RP11). Mol. Cell. 2001; 8:375–381. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

