Feasibility and Acceptability of a Task-Shifted Intervention to Enhance Adherence to HIV Medication and Improve Depression in People Living with HIV in Zimbabwe, a Low Income Country in Sub-Saharan Africa
- PMID: 28063075
- PMCID: PMC5758696
- DOI: 10.1007/s10461-016-1659-4
Feasibility and Acceptability of a Task-Shifted Intervention to Enhance Adherence to HIV Medication and Improve Depression in People Living with HIV in Zimbabwe, a Low Income Country in Sub-Saharan Africa
Abstract
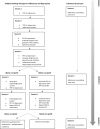
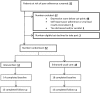
Using a pilot trial design in an HIV care clinic in Zimbabwe, we randomised 32 adults with poor adherence to antiretroviral therapy and at least mild depression to either six sessions of Problem-Solving Therapy for adherence and depression (PST-AD) delivered by an adherence counsellor, or to Enhanced Usual Care (Control). Acceptability of PST-AD was high, as indicated by frequency of session attendance and through qualitative analyses of exit interviews. Fidelity was >80% for the first two sessions of PST-AD but fidelity to the adherence component of PST-AD dropped by session 4. Contamination occurred, in that seven patients in the control arm received one or two PST-AD sessions before follow-up assessment. Routine health records proved unreliable for measuring HIV viral load at follow-up. Barriers to measuring adherence electronically included device failure and participant perception of being helped by the research device. The study was not powered to detect clinical differences, however, promising change at 6-months follow-up was seen in electronic adherence, viral load suppression (PST-AD arm 9/12 suppressed; control arm 4/8 suppressed) and depression (Patient Health Questionnaire-4.7 points in PST-AD arm vs. control, adjusted p value = 0.01). Results inform and justify a future randomised controlled trial of task-shifted PST-AD.
Keywords: Adherence; Depression; Intervention; Problem solving; Sub-Saharan Africa.
Conflict of interest statement
Conflict of interest
Dr. Steven Safren receives royalties from Oxford University Press and Guilford Publications for books on cognitive-behavioral treatments for various psychological difficulties, including coping with chronic illness and depression. All remaining author, Abas, Nyamayaro, Bere, Saruchera, Mothobi, Simms, Mangezi, Macpherson Croome, Magidson, Makadzange, Chibanda, O’Cleirigh declares that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. The study was approved by, the Medical Research Council of Zimbabwe and the Research Council of Zimbabwe (MRCZ/A/1736), Joint Parirenyatwa Hospital and College of Health Sciences Research Ethics Committee (JREC 18/13) and King’s College London College Research Ethics Committee (PNM/13/14-158). The trial was also registered with the Pan African Clinical Trials Registry (PACTR201511001150307).
Informed consent
Informed consent was obtained from all individual participants included in the study. Participants were compensated with USD3 per visit for public transport costs and were provided with refreshments.
Figures
References
-
- UNAIDS. Fact Sheet 2016. 2016.
-
- (UNAIDS) JUNPoHA. HIV and AIDS estimates (2015) 2015 http://www.unaids.org/en/regionscountries/countries/zimbabwe.
-
- ICAP. Zimbabwe Population-Based HIV Impact Assessment (2015–2016). 2016.
-
- UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. 2014.
-
- Kobin AB, Sheth NU. Levels of adherence required for virologic suppression among newer antiretroviral medications. Ann Pharmacother. 2011;45(3):372–379. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical