Long-term effectiveness of plasma-derived hepatitis B vaccine 22-28 years after immunization in a hepatitis B virus endemic rural area: is an adult booster dose needed?
- PMID: 28065199
- PMCID: PMC9507816
- DOI: 10.1017/S0950268816003046
Long-term effectiveness of plasma-derived hepatitis B vaccine 22-28 years after immunization in a hepatitis B virus endemic rural area: is an adult booster dose needed?
Abstract
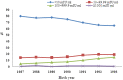
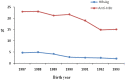
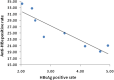
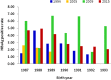
Longan County is considered a highly endemic area for hepatitis B virus (HBV). The plasma-derived vaccine has been used in newborns in this area since 1987. A cross-sectional survey was conducted to evaluate the long-term effectiveness of this vaccine. In total, 1634 participants born during 1987-1993 and who had received a series of plasma-derived HB vaccinations at ages 0, 1, and 6 months were enrolled. Serological HBV markers were detected and compared with previous survey data. Overall the prevalence of hepatitis B surface antigen (HBsAg) in all participants was 3·79%; 3·47% of subjects who had received the first dose within 24 h were HBsAg positive, and 8·41% of subjects who had received a delayed first dose were also HBsAg positive. There were 1527 subjects identified who had received the first dose within 24 h and whose HBsAg and anti-HBc prevalence increased yearly after immunization, while the anti-HBs-positive rate and vaccine effectiveness declined. The geometric mean concentration of antibody in the anti-HB-positive participants was 55·13 mIU/ml and this declined after immunization. Fewer than 2·0% of participants had anti-HB levels ⩾1000 mIU/ml. The data show that the protective efficacy of the plasma-derived vaccinations declined and administration of HB vaccine within 24 h of birth was very important. To reduce the risk of HBV infection in this highly endemic area, a booster dose might be necessary if anti-HBs levels fall below 10 mIU/ml after age 18 years. Furthermore, studies on the immune memory induced by plasma-derived HB vaccine are needed.
Keywords: Hepatitis B virus; long-term effectiveness; plasma-derived hepatitis B vaccine; primary immunization.
Conflict of interest statement
None.
Figures
References
-
- WHO. Hepatitis B fact sheet, 2015 (http://www.who.int/mediacentre/factsheets/fs204/en/index.html).
-
- Chang MH. Cancer prevention by vaccination against hepatitis B. Recent Results in Cancer Research 2009; 181: 85–94. - PubMed
-
- Ayoola AE, et al. The decline of hepatitis B viral infection in South-Western Saudi Arabia. Saudi Medical Journal 2003; 24: 991–995. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

