Calcium permeability of transient receptor potential canonical (TRPC) 4 channels measured by TRPC4-GCaMP6s
- PMID: 28066150
- PMCID: PMC5214905
- DOI: 10.4196/kjpp.2017.21.1.133
Calcium permeability of transient receptor potential canonical (TRPC) 4 channels measured by TRPC4-GCaMP6s
Abstract
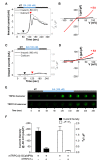
Conflicting evidence has been obtained regarding whether transient receptor potential cation channels (TRPC) are store-operated channels (SOCs) or receptor-operated channels (ROCs). Moreover, the Ca/Na permeability ratio differs depending on whether the current-voltage (I-V) curve has a doubly rectifying shape or inward rectifying shape. To investigate the calcium permeability of TRPC4 channels, we attached GCaMP6s to TRPC4 and simultaneously measured the current and calcium signals. A TRPC4 specific activator, (-)-englerin A, induced both current and calcium fluorescence with the similar time course. Muscarinic receptor stimulator, carbachol, also induced both current and calcium fluorescence with the similar time course. By forming heteromers with TRPC4, TRPC1 significantly reduced the inward current with outward rectifying I-V curve, which also caused the decrease of calcium fluorescence intensity. These results suggest that GCaMP6s attached to TRPC4 can detect slight calcium changes near TRPC4 channels. Consequently, TRPC4-GCaMP6s can be a useful tool for testing the calcium permeability of TRPC4 channels.
Keywords: Calcium; GCaMP6s; Receptor-operated channels; TRPC1/4 heteromer; TRPC4.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Pore residues of transient receptor potential channels canonical 1 and 4 heteromer determine channel properties.Am J Physiol Cell Physiol. 2023 Jul 1;325(1):C42-C51. doi: 10.1152/ajpcell.00488.2022. Epub 2023 May 22. Am J Physiol Cell Physiol. 2023. PMID: 37212545
-
Ca2+ signaling, TRP channels, and endothelial permeability.Microcirculation. 2006 Dec;13(8):693-708. doi: 10.1080/10739680600930347. Microcirculation. 2006. PMID: 17085428 Review.
-
TRPC4 and TRPC5: receptor-operated Ca2+-permeable nonselective cation channels.Cell Calcium. 2003 May-Jun;33(5-6):441-50. doi: 10.1016/s0143-4160(03)00055-1. Cell Calcium. 2003. PMID: 12765689 Review.
-
Role of Ca2+ signaling in the regulation of endothelial permeability.Vascul Pharmacol. 2002 Nov;39(4-5):173-85. doi: 10.1016/s1537-1891(03)00007-7. Vascul Pharmacol. 2002. PMID: 12747958 Review.
-
Tonantzitlolone is a nanomolar potency activator of transient receptor potential canonical 1/4/5 channels.Br J Pharmacol. 2018 Aug;175(16):3361-3368. doi: 10.1111/bph.14379. Epub 2018 Jun 28. Br J Pharmacol. 2018. PMID: 29859013 Free PMC article.
Cited by
-
Identification of phospholipase C β downstream effect on transient receptor potential canonical 1/4, transient receptor potential canonical 1/5 channels.Korean J Physiol Pharmacol. 2019 Sep;23(5):357-366. doi: 10.4196/kjpp.2019.23.5.357. Epub 2019 Aug 26. Korean J Physiol Pharmacol. 2019. PMID: 31496873 Free PMC article.
-
Dual action of the Gαq-PLCβ-PI(4,5)P2 pathway on TRPC1/4 and TRPC1/5 heterotetramers.Sci Rep. 2018 Aug 14;8(1):12117. doi: 10.1038/s41598-018-30625-0. Sci Rep. 2018. PMID: 30108272 Free PMC article.
-
Differential PI(4,5)P2 sensitivities of TRPC4, C5 homomeric and TRPC1/4, C1/5 heteromeric channels.Sci Rep. 2019 Feb 12;9(1):1849. doi: 10.1038/s41598-018-38443-0. Sci Rep. 2019. PMID: 30755645 Free PMC article.
-
Plasma Membrane Localized GCaMP-MS4A12 by Orai1 Co-Expression Shows Thapsigargin- and Ca2+-Dependent Fluorescence Increases.Mol Cells. 2021 Apr 30;44(4):223-232. doi: 10.14348/molcells.2021.2031. Mol Cells. 2021. PMID: 33935043 Free PMC article.
-
Role of Transient Receptor Potential Canonical Channels in Heart Physiology and Pathophysiology.Front Cardiovasc Med. 2020 Feb 25;7:24. doi: 10.3389/fcvm.2020.00024. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 32158769 Free PMC article. Review.
References
-
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. - PubMed
-
- Nilius B, Voets T. TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflugers Arch. 2005;451:1–10. - PubMed
-
- Putney JW. Physiological mechanisms of TRPC activation. Pflugers Arch. 2005;451:29–34. - PubMed
-
- Brough GH, Wu S, Cioffi D, Moore TM, Li M, Dean N, Stevens T. Contribution of endogenously expressed Trp1 to a Ca2+-selective, store-operated Ca2+ entry pathway. FASEB J. 2001;15:1727–1738. - PubMed
-
- Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4-/- mice. Nat Cell Biol. 2001;3:121–127. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

