Early Cognitive/Social Deficits and Late Motor Phenotype in Conditional Wild-Type TDP-43 Transgenic Mice
- PMID: 28066234
- PMCID: PMC5167738
- DOI: 10.3389/fnagi.2016.00310
Early Cognitive/Social Deficits and Late Motor Phenotype in Conditional Wild-Type TDP-43 Transgenic Mice
Abstract
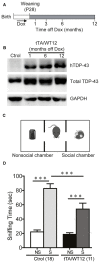
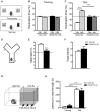
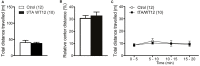
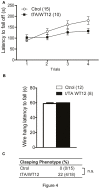
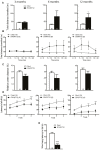
Frontotemporal Dementia (FTD) and amyotrophic lateral sclerosis (ALS) are two neurodegenerative diseases associated to mislocalization and aggregation of TAR DNA-binding protein 43 (TDP-43). To investigate in depth the behavioral phenotype associated with this proteinopathy, we used as a model transgenic (Tg) mice conditionally overexpressing human wild-type TDP 43 protein (hTDP-43-WT) in forebrain neurons. We previously characterized these mice at the neuropathological level and found progressive neurodegeneration and other features that evoke human TDP-43 proteinopathies of the FTD/ALS spectrum. In the present study we analyzed the behavior of mice at multiple domains, including motor, social and cognitive performance. Our results indicate that young hTDP-43-WT Tg mice (1 month after post-weaning transgene induction) present a normal motor phenotype compared to control littermates, as assessed by accelerated rotarod performance, spontaneous locomotor activity in the open field test and a mild degree of spasticity shown by a clasping phenotype. Analysis of social and cognitive behavior showed a rapid installment of deficits in social interaction, working memory (Y-maze test) and recognition memory (novel object recognition test) in the absence of overt motor abnormalities. To investigate if the motor phenotype worsen with age, we analyzed the behavior of mice after long-term (up to 12 months) transgene induction. Our results reveal a decreased performance on the rotarod test and in the hanging wire test, indicating a motor phenotype that was absent in younger mice. In addition, long-term hTDP-43-WT expression led to hyperlocomotion in the open field test. In sum, these results demonstrate a time-dependent emergence of a motor phenotype in older hTDP-43-WT Tg mice, recapitulating aspects of clinical FTD presentations with motor involvement in human patients, and providing a complementary animal model for studying TDP-43 proteinopathies.
Keywords: TDP-43; amyotrophic lateral sclerosis; animal model; behavior; frontotemporal dementia; proteinopathy; transgenic mice.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

