Antinociceptive Effects of AGAP, a Recombinant Neurotoxic Polypeptide: Possible Involvement of the Tetrodotoxin-Resistant Sodium Channels in Small Dorsal Root Ganglia Neurons
- PMID: 28066245
- PMCID: PMC5168466
- DOI: 10.3389/fphar.2016.00496
Antinociceptive Effects of AGAP, a Recombinant Neurotoxic Polypeptide: Possible Involvement of the Tetrodotoxin-Resistant Sodium Channels in Small Dorsal Root Ganglia Neurons
Abstract
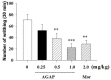
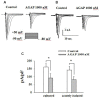
Antitumor-analgesic peptide (AGAP) is a novel recombinant polypeptide. The primary study showed that AGAP 1.0 mg/kg exhibited strong analgesic and antitumor effects. The tail vein administration of AGAP potently reduced pain behaviors in mice induced by intraplantar injection of formalin or intraperitoneal injection of acetic acid, without affecting basal pain perception. To further assess the mechanisms of AGAP, the effects of AGAP on sodium channels were assessed using the whole-cell patch clamp recordings in dorsal root ganglia (DRG) neurons. The results showed that AGAP (3-1000 nM) inhibited the sodium currents in small-diameter DRG neurons in a dose-dependent manner. 1000 nM AGAP could inhibit the current density-voltage relationship curve of sodium channels in a voltage-dependent manner and negatively shift the activation curves. 1000 nM AGAP could reduce the tetrodotoxin-resistant (TTX-R) sodium currents by 42.8% in small-diameter DRG neurons. Further analysis revealed that AGAP potently inhibited NaV1.8 currents by 59.4%, and negatively shifted the activation and inactivation kinetics. 1000 nM AGAP also reduced the NaV1.9 currents by 33.7%, but had no significant effect on activation and inactivation kinetics. Thus, our results demonstrated that submicromolar concentrations of AGAP inhibited TTX-R sodium channel in rat small-diameter DRG neurons. It is concluded that these new results may better explain, at least in part, the analgesic properties of this polypeptide.
Keywords: Nav1.8; Nav1.9; Voltage-gated sodium channels; pain; scorpion toxins.
Figures







References
LinkOut - more resources
Full Text Sources
Other Literature Sources

