Recognizing the Common Origins of Dystonia and the Development of Human Movement: A Manifesto of Unmet Needs in Isolated Childhood Dystonias
- PMID: 28066314
- PMCID: PMC5165260
- DOI: 10.3389/fneur.2016.00226
Recognizing the Common Origins of Dystonia and the Development of Human Movement: A Manifesto of Unmet Needs in Isolated Childhood Dystonias
Abstract
Dystonia in childhood may be severely disabling and often unremitting and unrecognized. Considered a rare disorder, dystonic symptoms in childhood are pervasive in many conditions including disorders of developmental delay, cerebral palsy (CP), autism, neurometabolic, neuroinflammatory, and neurogenetic disorders. Collectively, there is a need to recognize the role of early postures and movements which characterize phases of normal fetal, infant, and child development as a backdrop to the many facets of dystonia in early childhood neurological disorders and to be aware of the developmental context of dystonic symptoms. The role of cocontraction is explored throughout infancy, childhood, young adulthood, and in the elderly. Under-recognition of pervasive dystonic disorders of childhood, including within CP is reviewed. Original descriptions of CP by Gowers are reviewed and contemporary physiological demonstrations are used to illustrate support for an interpretation of the tonic labyrinthine response as a manifestation of dystonia. Early recognition and molecular diagnosis of childhood dystonia where possible are desirable for appropriate clinical stratification and future precision medicine and functional neurosurgery where appropriate. A developmental neurobiological perspective could also be useful in exploring new clinical strategies for adult-onset dystonia disorders focusing on environmental and molecular interactions and systems behaviors.
Keywords: Gowers; cerebral palsy; cocontraction; developmental dystonia; dystonia; genetic heterogeneity; phenotypic pleiotropy; tonic labyrinthine response.
Figures

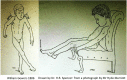

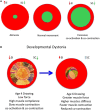

References
-
- Moessinger AC. Fetal akinesia deformation sequence: an animal model. Pediatrics (1983) 72:857–63. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

