Targeting Innate-Like T Cells in Tuberculosis
- PMID: 28066410
- PMCID: PMC5175204
- DOI: 10.3389/fimmu.2016.00594
Targeting Innate-Like T Cells in Tuberculosis
Abstract
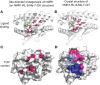
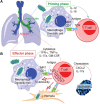
Peptide-specific conventional T cells have been major targets for designing most antimycobacterial vaccines. Immune responses mediated by conventional T cells exhibit a delayed onset upon primary infection and are highly variable in different human populations. In contrast, innate-like T cells quickly respond to pathogens and display effector functions without undergoing extensive clonal expansion. Specifically, the activation of innate-like T cells depends on the promiscuous interaction of highly conserved antigen-presenting molecules, non-peptidic antigens, and likely semi-invariant T cell receptors. In antimicrobial immune responses, mucosal-associated invariant T cells are activated by riboflavin precursor metabolites presented by major histocompatibility complex-related protein I, while lipid-specific T cells including natural killer T cells are activated by lipid metabolites presented by CD1 proteins. Multiple innate-like T cell subsets have been shown to be protective or responsive in mycobacterial infections. Through rapid cytokine secretion, innate-like T cells function in early defense and memory response, offering novel advantages over conventional T cells in the design of anti-tuberculosis strategies.
Keywords: CD1; MR1; Mycobacterium tuberculosis; antigen presentation; innate-like T cells; lipid; riboflavin metabolites; vaccine.
Figures



References
-
- Orme IM, Collins FM. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol (1984) 84(1):113–20. 10.1016/0008-8749(84)90082-0 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

