Complement Protein C1q Interacts with DC-SIGN via Its Globular Domain and Thus May Interfere with HIV-1 Transmission
- PMID: 28066413
- PMCID: PMC5177617
- DOI: 10.3389/fimmu.2016.00600
Complement Protein C1q Interacts with DC-SIGN via Its Globular Domain and Thus May Interfere with HIV-1 Transmission
Abstract
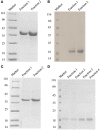
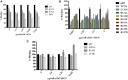
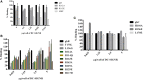
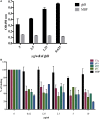
Dendritic cells (DCs) are the most potent antigen-presenting cells capable of priming naïve T-cells. Its C-type lectin receptor, DC-SIGN, regulates a wide range of immune functions. Along with its role in HIV-1 pathogenesis through complement opsonization of the virus, DC-SIGN has recently emerged as an adaptor for complement protein C1q on the surface of immature DCs via a trimeric complex involving gC1qR, a receptor for the globular domain of C1q. Here, we have examined the nature of interaction between C1q and DC-SIGN in terms of domain localization, and implications of C1q-DC-SIGN-gC1qR complex formation on HIV-1 transmission. We first expressed and purified recombinant extracellular domains of DC-SIGN and its homologue DC-SIGNR as tetramers comprising of the entire extra cellular domain including the α-helical neck region and monomers comprising of the carbohydrate recognition domain only. Direct binding studies revealed that both DC-SIGN and DC-SIGNR were able to bind independently to the recombinant globular head modules ghA, ghB, and ghC, with ghB being the preferential binder. C1q appeared to interact with DC-SIGN or DC-SIGNR in a manner similar to IgG. Mutational analysis using single amino acid substitutions within the globular head modules showed that TyrB175 and LysB136 were critical for the C1q-DC-SIGN/DC-SIGNR interaction. Competitive studies revealed that gC1qR and ghB shared overlapping binding sites on DC-SIGN, implying that HIV-1 transmission by DCs could be modulated due to the interplay of gC1qR-C1q with DC-SIGN. Since C1q, gC1qR, and DC-SIGN can individually bind HIV-1, we examined how C1q and gC1qR modulated HIV-1-DC-SIGN interaction in an infection assay. Here, we report, for the first time, that C1q suppressed DC-SIGN-mediated transfer of HIV-1 to activated pooled peripheral blood mononuclear cells, although the globular head modules did not. The protective effect of C1q was negated by the addition of gC1qR. In fact, gC1qR enhanced DC-SIGN-mediated HIV-1 transfer, suggesting its role in HIV-1 pathogenesis. Our results highlight the consequences of multiple innate immune pattern recognition molecules forming a complex that can modify their functions in a way, which may be advantageous for the pathogen.
Keywords: C1q; DC-SIGN; HIV-1; globular head domain; protein–protein interaction.
Figures



Similar articles
-
Analysis of the Interaction between Globular Head Modules of Human C1q and Its Candidate Receptor gC1qR.Front Immunol. 2016 Dec 13;7:567. doi: 10.3389/fimmu.2016.00567. eCollection 2016. Front Immunol. 2016. PMID: 28018340 Free PMC article.
-
DC-SIGN, C1q, and gC1qR form a trimolecular receptor complex on the surface of monocyte-derived immature dendritic cells.Blood. 2012 Aug 9;120(6):1228-36. doi: 10.1182/blood-2011-07-369728. Epub 2012 Jun 13. Blood. 2012. PMID: 22700724 Free PMC article.
-
Localization of ligand-binding sites on human C1q globular head region using recombinant globular head fragments and single-chain antibodies.Biochim Biophys Acta. 2003 Nov 3;1652(1):64-74. doi: 10.1016/j.bbapap.2003.08.003. Biochim Biophys Acta. 2003. PMID: 14580997
-
DC-SIGN (dendritic cell-specific ICAM-grabbing non-integrin) and DC-SIGN-related (DC-SIGNR): friend or foe?Clin Sci (Lond). 2003 Apr;104(4):437-46. Clin Sci (Lond). 2003. PMID: 12653690 Review.
-
The C1q Receptors: Focus on gC1qR/p33 (C1qBP, p32, HABP-1)1.Semin Immunol. 2019 Oct;45:101338. doi: 10.1016/j.smim.2019.101338. Epub 2019 Nov 16. Semin Immunol. 2019. PMID: 31744753 Review.
Cited by
-
gC1qR: A New Target for Cancer Immunotherapy.Front Immunol. 2023 Jan 26;14:1095943. doi: 10.3389/fimmu.2023.1095943. eCollection 2023. Front Immunol. 2023. PMID: 36776869 Free PMC article. Review.
-
Human surfactant protein D facilitates SARS-CoV-2 pseudotype binding and entry in DC-SIGN expressing cells, and downregulates spike protein induced inflammation.Front Immunol. 2022 Jul 28;13:960733. doi: 10.3389/fimmu.2022.960733. eCollection 2022. Front Immunol. 2022. PMID: 35967323 Free PMC article.
-
SLE: Novel Postulates for Therapeutic Options.Front Immunol. 2020 Oct 7;11:583853. doi: 10.3389/fimmu.2020.583853. eCollection 2020. Front Immunol. 2020. PMID: 33117397 Free PMC article. Review.
-
Complement Proteins as Soluble Pattern Recognition Receptors for Pathogenic Viruses.Viruses. 2021 May 2;13(5):824. doi: 10.3390/v13050824. Viruses. 2021. PMID: 34063241 Free PMC article. Review.
-
Innate Immune Response Against HIV-1.Adv Exp Med Biol. 2021;1313:23-58. doi: 10.1007/978-3-030-67452-6_3. Adv Exp Med Biol. 2021. PMID: 34661890
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

