Chemoradiation Increases PD-L1 Expression in Certain Melanoma and Glioblastoma Cells
- PMID: 28066420
- PMCID: PMC5177615
- DOI: 10.3389/fimmu.2016.00610
Chemoradiation Increases PD-L1 Expression in Certain Melanoma and Glioblastoma Cells
Abstract
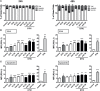
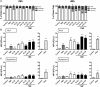
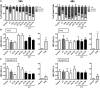
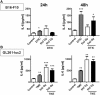
Immunotherapy approaches currently make their way into the clinics to improve the outcome of standard radiochemotherapy (RCT). The programed cell death receptor ligand 1 (PD-L1) is one possible target that, upon blockade, allows T cell-dependent antitumor immune responses to be executed. To date, it is unclear which RCT protocol and which fractionation scheme leads to increased PD-L1 expression and thereby renders blockade of this immune suppressive pathway reasonable. We therefore investigated the impact of radiotherapy (RT), chemotherapy (CT), and RCT on PD-L1 surface expression on tumor cells of tumor entities with differing somatic mutation prevalence. Murine melanoma (B16-F10), glioblastoma (GL261-luc2), and colorectal (CT26) tumor cells were treated with dacarbazine, temozolomide, and a combination of irinotecan, oxaliplatin, and fluorouracil, respectively. Additionally, they were irradiated with a single dose [10 Gray (Gy)] or hypo-fractionated (2 × 5 Gy), respectively, norm-fractionated (5 × 2 Gy) radiation protocols were used. PD-L1 surface and intracellular interferon (IFN)-gamma expression was measured by flow cytometry, and IL-6 release was determined by ELISA. Furthermore, tumor cell death was monitored by AnnexinV-FITC/7-AAD staining. For first in vivo analyses, the B16-F10 mouse melanoma model was chosen. In B16-F10 and GL261-luc2 cells, particularly norm-fractionated and hypo-fractionated radiation led to a significant increase of surface PD-L1, which could not be observed in CT26 cells. Furthermore, PD-L1 expression is more pronounced on vital tumor cells and goes along with increased levels of IFN-gamma in the tumor cells. In melanoma cells CT was the main trigger for IL-6 release, while in glioblastoma cells it was norm-fractionated RT. In vivo, fractionated RT only in combination with dacarbazine induced PD-L1 expression on melanoma cells. Our results suggest a tumor cell-mediated upregulation of PD-L1 expression following in particular chemoradiation that is not only dependent on the somatic mutation prevalence of the tumor entity.
Keywords: IFN-gamma; IL-6; PD-L1; checkpoint inhibitor; fractionated radiotherapy; glioblastoma; immunotherapy; melanoma.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

