Complement Evasion by Pathogenic Leptospira
- PMID: 28066433
- PMCID: PMC5174078
- DOI: 10.3389/fimmu.2016.00623
Complement Evasion by Pathogenic Leptospira
Abstract
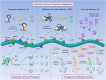
Leptospirosis is a neglected infectious disease caused by spirochetes from the genus Leptospira. Pathogenic microorganisms, notably those which reach the blood circulation such as Leptospira, have evolved multiple strategies to escape the host complement system, which is important for innate and acquired immunity. Leptospira avoid complement-mediated killing through: (i) recruitment of host complement regulators; (ii) acquisition of host proteases that cleave complement proteins on the bacterial surface; and, (iii) secretion of proteases that inactivate complement proteins in the Leptospira surroundings. The recruitment of host soluble complement regulatory proteins includes the acquisition of Factor H (FH) and FH-like-1 (alternative pathway), C4b-binding protein (C4BP) (classical and lectin pathways), and vitronectin (Vn) (terminal pathway). Once bound to the leptospiral surface, FH and C4BP retain cofactor activity of Factor I in the cleavage of C3b and C4b, respectively. Vn acquisition by leptospires may result in terminal pathway inhibition by blocking C9 polymerization. The second evasion mechanism lies in plasminogen (PLG) binding to the leptospiral surface. In the presence of host activators, PLG is converted to enzymatically active plasmin, which is able to degrade C3b, C4b, and C5 at the surface of the pathogen. A third strategy used by leptospires to escape from complement system is the active secretion of proteases. Pathogenic, but not saprophytic leptospires, are able to secrete metalloproteases that cleave C3 (central complement molecule), Factor B (alternative pathway), and C4 and C2 (classical and lectin pathways). The purpose of this review is to fully explore these complement evasion mechanisms, which act together to favor Leptospira survival and multiplication in the host.
Keywords: Leptospira; complement system; immune evasion; leptospirosis; serum resistance.
Figures
References
-
- Faine S, Adler B, Bolin C, Perolat P. Leptospira and Leptospirosis. Melbourne: MedScience; (1999).
-
- Levett PN. Systematics of leptospiraceae. In: Adler B, editor. Leptospira and Leptospirosis. Current Topics in Microbiology and Immunology. New York: Springer-Verlag Berlin Heidelberg; (2015). p. 11–20. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

