Stepwise N-H Bond Formation From N2-Derived Iron Nitride, Imide and Amide Intermediates to Ammonia
- PMID: 28066537
- PMCID: PMC5207225
- DOI: 10.1039/C6SC00423G
Stepwise N-H Bond Formation From N2-Derived Iron Nitride, Imide and Amide Intermediates to Ammonia
Abstract
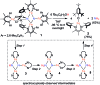
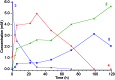
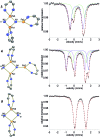
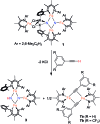
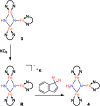
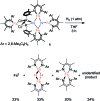
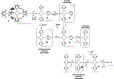
Reduction of N2 to ammonia in nature and in electrocatalysis takes place through 1-proton/1-electron steps, motivating efforts to experimentally study the steps during proton/electron transfer to well-characterized N2-derived species with bridging nitrides. We report here the protonation and reduction reactions of an N2-derived iron bis(nitride) complex (Rodriguez et al., Science, 2011, 334, 780). We isolate and definitively characterize triiron imido and amido intermediates that lie along the path to ammonia formation, and Mössbauer spectroscopy shows the oxidation level of iron atoms in these mixed-valence clusters. The first two H atoms add to one of the two nitrides of the bis(nitride) complex, and the proton-coupled electron transfer in the second step can be concerted or stepwise depending on the sources of protons and electrons. The characterization of partially protonated nitrides and their mechanisms of formation are expected to guide efforts to convert N2 to ammonia with mild acids.
Figures

References
-
- Smil V. Sci. Am. 1997;277:76–81.
- Jenkinson D. S. Plant Soil. 2001;228:3–15.
-
- Mittasch A., Geschichte der Ammoniaksynthese, Verlag Chemie, Weinheim, 1951.
- Ammonia: Catalysis and Manufacture, Nielsen (ed.), Springer, Berlin, 1995.
- Schlögl R. Angew. Chem., Int. Ed. 2003;42:2004–2008. - PubMed
-
- Schlögl R., in Handbook of Heterogeneous Catalysis, Wiley-VCH, Weinheim, 2nd edn, 2008, vol. 5, pp. 2501–2575.
-
- Rees D. C., Howard J. B. Science. 2003;300:929–931. - PubMed
- Spatzal T., Aksoyoglu M., Zhang L., Andrade S. L. A., Schleicher E., Weber S., Rees D. C., Einsle O. Science. 2011;334:940. - PMC - PubMed
- Lancaster K. M., Roemelt M., Ettenhuber P., Hu Y., Ribbe M. W., Neese F., Bergmann U., DeBeer S. Science. 2011;334:974–977. - PMC - PubMed
- Eady R. R. Chem. Rev. 1996;96:3013–3030. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

