A microfluidic-based cell encapsulation platform to achieve high long-term cell viability in photopolymerized PEGNB hydrogel microspheres
- PMID: 28066550
- PMCID: PMC5207045
- DOI: 10.1039/C6TB02551J
A microfluidic-based cell encapsulation platform to achieve high long-term cell viability in photopolymerized PEGNB hydrogel microspheres
Abstract
Cell encapsulation within photopolymerized polyethylene glycol (PEG)-based hydrogel scaffolds has been demonstrated as a robust strategy for cell delivery, tissue engineering, regenerative medicine, and developing in vitro platforms to study cellular behavior and fate. Strategies to achieve spatial and temporal control over PEG hydrogel mechanical properties, chemical functionalization, and cytocompatibility have advanced considerably in recent years. Recent microfluidic technologies have enabled the miniaturization of PEG hydrogels, thus enabling the fabrication of miniaturized cell-laden vehicles. However, rapid oxygen diffusive transport times on the microscale dramatically inhibit chain growth photopolymerization of polyethylene glycol diacrylate (PEGDA), thus decreasing the viability of cells encapsulated within these microstructures. Another promising PEG-based scaffold material, PEG norbornene (PEGNB), is formed by a step-growth photopolymerization and is not inhibited by oxygen. PEGNB has also been shown to be more cytocompatible than PEGDA and allows for orthogonal addition reactions. The step-growth kinetics, however, are slow and therefore challenging to fully polymerize within droplets flowing through microfluidic devices. Here, we describe a microfluidic-based droplet fabrication platform that generates consistently monodisperse cell-laden water-in-oil emulsions. Microfluidically generated PEGNB droplets are collected and photopolymerized under UV exposure in bulk emulsions. In this work, we compare this microfluidic-based cell encapsulation platform with a vortex-based method on the basis of microgel size, uniformity, post-encapsulation cell viability and long-term cell viability. Several factors that influence post-encapsulation cell viability were identified. Finally, long-term cell viability achieved by this platform was compared to a similar cell encapsulation platform using PEGDA. We show that this PEGNB microencapsulation platform is capable of generating cell-laden hydrogel microspheres at high rates with well-controlled size distributions and high long-term cell viability.
Figures
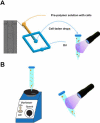
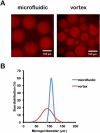



References
-
- Bryant SJ, Bender RJ, Durand KL, Anseth KS. Biotechnol. Bioeng. 2004;86:747–755. - PubMed
-
- Lacy PE, Hegre OD, Gerasimidi-Vazeou A, GENTILE FT, Dionne KE. Science. 1991;254:1782–1784. - PubMed
-
- Murua A, Portero A, Orive G, Hernández RM, de Castro M, Pedraz JL. Journal of Controlled Release. 2008;132:76–83. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

