Expression of genes involved in brain GABAergic neurotransmission in three-spined stickleback exposed to near-future CO2
- PMID: 28066553
- PMCID: PMC5196030
- DOI: 10.1093/conphys/cow068
Expression of genes involved in brain GABAergic neurotransmission in three-spined stickleback exposed to near-future CO2
Erratum in
-
Erratum: Expression of genes involved in brain GABAergic neurotransmission in three-spined stickleback exposed to near-future CO2.Conserv Physiol. 2017 Jan 20;5(1):cox004. doi: 10.1093/conphys/cox004. eCollection 2017. Conserv Physiol. 2017. PMID: 28149521 Free PMC article.
Abstract
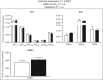
Change in the activity of the main inhibitory receptor, GABAA, has been suggested to be a general mechanism behind the behavioural alterations reported in ocean acidification studies on fish. It has been proposed that regulatory acid-base mechanisms in response to high CO2 alter the neuronal Cl- and HCO3- gradients that are important for GABAA receptor function. Here, we report a comprehensive analysis of gene expression of GABAA receptor subunits and of genes involved in GABAergic transmission in the brain of fish exposed to near-future CO2. Altogether, 56 mRNA transcripts were quantified in brains of three-spined stickleback (Gasterosteus aculeatus) kept in control pCO2 (333 ± 30 μatm CO2) or at high pCO2 levels (991 ± 57 μatm) for 43 days. The gene expression analysis included GABAA receptor subunits (α1-6, β1-3, γ1-3, δ, π and ρ1-3), enzymes and transporters involved in GABA metabolism (GAD1-2, GABAT and GAT1-3), GABAA receptor-associated proteins (GABARAP and GABARAPL), ion cotransporters (KCC1-4, NKCC1, ClC21-3, AE3 and NDAE) and carbonic anhydrase (CAII). Exposure to high CO2 had only minor effects on the expression of genes involved in GABAergic neurotransmission. There were significant increases in the mRNA levels of α family subunits of the GABAA receptor, with a more pronounced expression of α12, α3, α4 and α6b. No changes were detected in the expression of other GABAA subunits or in genes related to receptor turnover, GABA metabolism or ion transport. Although the minor changes seen for mRNA levels might reflect compensatory mechanisms in the high-CO2 conditions, these were apparently insufficient to restore normal neural function, because the behavioural changes persisted within the time frame studied.
Keywords: GABAA receptor; GABAergic system; ion cotranporters; ocean acidification; quantitative polymerase chain reaction; three-spined stickleback.
Figures



References
-
- Anzelius M, Ekström P, Möhler H, Richards JG (1995) Immunocytochemical localization of GABAA receptor β2/β3 subunits in the brain of Atlantic salmon (Salmo salar L). J Chem Neuroanat 8: 207–221. - PubMed
-
- Beerling DJ, Royer DL (2011) Convergent cenozoic CO2 history. Nat Geosci 4: 418–420.
-
- Benke D, Fakitsas P, Roggenmoser C, Michel C, Rudolph U, Mohler H (2004) Analysis of the presence and abundance of GABAA receptors containing two different types of α subunits in murine brain using point-mutated α subunits. J Biol Chem 279: 43654–43660. - PubMed
-
- Bloom FE, Iversen (1971) Localizing 3H-GABA in nerve terminals of rat cerebral cortex by electron microscopic autoradiography. Nature 229: 628–630. - PubMed
-
- Böhme I, Rabe H, Lüddens H (2004) Four amino acids in the α subunits determine the γ-aminobutyric acid sensitivities of GABAA receptor subtypes. J Biol Chem 279: 35193–35200. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

