Functional Intestinal Bile Acid 7α-Dehydroxylation by Clostridium scindens Associated with Protection from Clostridium difficile Infection in a Gnotobiotic Mouse Model
- PMID: 28066726
- PMCID: PMC5168579
- DOI: 10.3389/fcimb.2016.00191
Functional Intestinal Bile Acid 7α-Dehydroxylation by Clostridium scindens Associated with Protection from Clostridium difficile Infection in a Gnotobiotic Mouse Model
Abstract
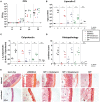
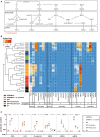
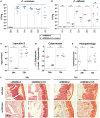
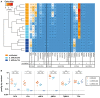
Bile acids, important mediators of lipid absorption, also act as hormone-like regulators and as antimicrobial molecules. In all these functions their potency is modulated by a variety of chemical modifications catalyzed by bacteria of the healthy gut microbiota, generating a complex variety of secondary bile acids. Intestinal commensal organisms are well-adapted to normal concentrations of bile acids in the gut. In contrast, physiological concentrations of the various intestinal bile acid species play an important role in the resistance to intestinal colonization by pathogens such as Clostridium difficile. Antibiotic therapy can perturb the gut microbiota and thereby impair the production of protective secondary bile acids. The most important bile acid transformation is 7α-dehydroxylation, producing deoxycholic acid (DCA) and lithocholic acid (LCA). The enzymatic pathway carrying out 7α-dehydroxylation is restricted to a narrow phylogenetic group of commensal bacteria, the best-characterized of which is Clostridium scindens. Like many other intestinal commensal species, 7-dehydroxylating bacteria are understudied in vivo. Conventional animals contain variable and uncharacterized indigenous 7α-dehydroxylating organisms that cannot be selectively removed, making controlled colonization with a specific strain in the context of an undisturbed microbiota unfeasible. In the present study, we used a recently established, standardized gnotobiotic mouse model that is stably associated with a simplified murine 12-species "oligo-mouse microbiota" (Oligo-MM12). It is representative of the major murine intestinal bacterial phyla, but is deficient for 7α-dehydroxylation. We find that the Oligo-MM12 consortium carries out bile acid deconjugation, a prerequisite for 7α-dehydroxylation, and confers no resistance to C. difficile infection (CDI). Amendment of Oligo-MM12 with C. scindens normalized the large intestinal bile acid composition by reconstituting 7α-dehydroxylation. These changes had only minor effects on the composition of the native Oligo-MM12, but significantly decreased early large intestinal C. difficile colonization and pathogenesis. The delayed pathogenesis of C. difficile in C. scindens-colonized mice was associated with breakdown of cecal microbial bile acid transformation.
Keywords: 7α-dehydroxylation; Clostridium difficile; Clostridium difficile infection (CDI); Clostridium scindens; gnotobiotic mouse model; gut microbiota; intestinal infection; secondary bile acids.
Figures
References
-
- Barthel M., Hapfelmeier S., Quintanilla-Martinez L., Kremer M., Rohde M., Hogardt M., et al. (2003). Pretreatment of mice with streptomycin provides a Salmonella enterica serovar typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71:2839. 10.1128/IAI.71.5.2839-2858.2003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

