Short-acting erythropoiesis-stimulating agents for anaemia in predialysis patients
- PMID: 28066881
- PMCID: PMC6464724
- DOI: 10.1002/14651858.CD011690.pub2
Short-acting erythropoiesis-stimulating agents for anaemia in predialysis patients
Abstract
Background: The benefits of erythropoiesis-stimulating agents (ESA) for chronic kidney disease (CKD) patients have been previously demonstrated. However, the efficacy and safety of short-acting epoetins administered at larger doses and reduced frequency as well as of new epoetins and biosimilars remains uncertain.
Objectives: This review aimed to evaluate the benefits and harms of different routes, frequencies and doses of epoetins (epoetin alpha, epoetin beta and other short-acting epoetins) for anaemia in adults and children with CKD not receiving dialysis.
Search methods: We searched the Cochrane Kidney and Transplant Specialised Register to 12 September 2016 through contact with the Information Specialist using search terms relevant to this review. Studies contained in the Specialised Register are identified through search strategies specifically designed for CENTRAL, MEDLINE, and EMBASE; handsearching conference proceedings; and searching the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria: We included randomised control trials (RCTs) comparing different frequencies, routes, doses and types of short-acting ESAs in CKD patients.
Data collection and analysis: Two authors independently assessed study eligibility and four authors assessed risk of bias and extracted data. Results were expressed as risk ratio (RR) or risk differences (RD) with 95% confidence intervals (CI) for dichotomous outcomes. For continuous outcomes the mean difference (MD) with 95% confidence intervals (CI) was used. Statistical analyses were performed using the random-effects model.
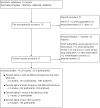
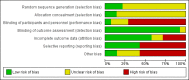
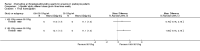
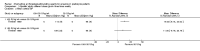
Main results: We identified 14 RCTs (2616 participants); nine studies were multi-centre and two studies involved children. The risk of bias was high in most studies; only three studies demonstrated adequate random sequence generation and only two studies were at low risk of bias for allocation concealment. Blinding of participants and personnel was at low risk of bias in one study. Blinding of outcome assessment was judged at low risk in 13 studies as the outcome measures were reported as laboratory results and therefore unlikely to be influenced by blinding. Attrition bias was at low risk of bias in eight studies while selective reporting was at low risk in six included studies.Four interventions were compared: epoetin alpha or beta at different frequencies using the same total dose (six studies); epoetin alpha at the same frequency and different total doses (two studies); epoetin alpha administered intravenously versus subcutaneous administration (one study); epoetin alpha or beta versus other epoetins or biosimilars (five studies). One study compared both different frequencies of epoetin alpha at the same total dose and at the same frequency using different total doses.Data from only 7/14 studies could be included in our meta-analyses. There were no significant differences in final haemoglobin (Hb) levels when dosing every two weeks was compared with weekly dosing (4 studies, 785 participants: MD -0.20 g/dL, 95% CI -0.33 to -0.07), when four weekly dosing was compared with two weekly dosing (three studies, 671 participants: MD -0.16 g/dL, 95% CI -0.43 to 0.10) or when different total doses were administered at the same frequency (four weekly administration: one study, 144 participants: MD 0.17 g/dL 95% CI -0.19 to 0.53).Five studies evaluated different interventions. One study compared epoetin theta with epoetin alpha and found no significant differences in Hb levels (288 participants: MD -0.02 g/dL, 95% CI -0.25 to 0.21). One study found significantly higher pain scores with subcutaneous epoetin alpha compared with epoetin beta. Two studies (165 participants) compared epoetin delta with epoetin alpha, with no results available since the pharmaceutical company withdrew epoetin delta for commercial reasons. The fifth study comparing the biosimilar HX575 with epoetin alpha was stopped after patients receiving HX575 subcutaneously developed anti-epoetin antibodies and no results were available.Adverse events were poorly reported in all studies and did not differ significantly within comparisons. Mortality was only detailed adequately in four studies and only one study included quality of life data.
Authors' conclusions: Epoetin alpha given at higher doses for extended intervals (two or four weekly) is non-inferior to more frequent dosing intervals in maintaining final Hb levels with no significant differences in adverse effects in non-dialysed CKD patients. However the data are of low methodological quality so that differences in efficacy and safety cannot be excluded. Further large, well designed, RCTs with patient-centred outcomes are required to assess the safety and efficacy of large doses of the shorter acting ESAs, including biosimilars of epoetin alpha, administered less frequently compared with more frequent administration of smaller doses in children and adults with CKD not on dialysis.
Conflict of interest statement
Deirdre Hahn: none known
Elisabeth M Hodson: none known
Angela C Webster: none known
Noha Elserafy: none known
Christopher Esezobor: none known
Figures









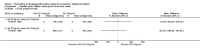
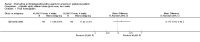
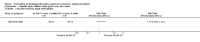




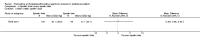


Update of
- doi: 10.1002/14651858.CD011690
References
References to studies included in this review
Aggarwal 2002 {published data only}
-
- Aggarwal HK, Kaushik G, Singh S, Nand N, Singh M. Comparison of efficacy of subcutaneous versus intravenous erythropoeitin in anaemia of chronic renal failure [abstract no: 227]. Journal of the Association of Physicians of India 2001;49(Jan). [CENTRAL: CN‐01094842]
-
- Aggarwal HK, Nand N, Singh S, Singh M, Kaushik G. Comparative efficacy of subcutaneous versus intravenous dose of erythropoietin in pre‐dialysis patients of chronic renal failure. Journal of Indian Academy of Clinical Medicine 2002;3(1):46‐50. [CENTRAL: CN‐00689631]
Akiba 1992 {published data only}
-
- Akiba T, Koshikawa S, Mimura N, Maeda T, KRN5702 Pre‐Dialysis Study Group. Randomized double‐blind study of subcutaneous recombinant human erythropoietin in predialysis uraemic patients [abstract]. Nephrology Dialysis Transplantation 1992;7(7):765. [CENTRAL: CN‐00260735]
Amon 1992 {published data only}
-
- Amon O, Muller‐Wiefel DE, Daniel K, Eife R, Leititis J, Stolpe HJ. Is S.C. erythropoietin (rhEPO) treatment thrice per week superior to an application once per week? [abstract]. 9th Congress. International Pediatric Nephrology Association; 1992 Aug 30 ‐ Sep 4; Jerusalem, Israel. 1992:C143. [CENTRAL: CN‐00483004]
Frenken 1989 {published data only}
-
- Frenken LA, Verberckmoes R, Michielsen P, Koene RA. Efficacy and tolerance of treatment with recombinant‐human erythropoietin in chronic renal failure (pre‐dialysis) patients. Nephrology Dialysis Transplantation 1989;4(9):782‐6. [MEDLINE: ] - PubMed
-
- Frenken LA, Verberckmoes R, Sluiter HE, Schrijver G, Michielsen P, Koene RA. An open study of the safety and efficacy of multiple doses of recombinant human erythropoietin in end‐stage renal disease (predialysis) patients [abstract]. Nephrology Dialysis Transplantation 1988;3(4):495. [CENTRAL: CN‐00260377]
-
- Frenken LA, Verberckmoes R, Sluiter HE, Schrijver G, Michielsen P, Koene RA. An open study of the safety and efficacy of multiple doses of recombinant‐human erythropoietin in end‐state renal disease (pre‐dialysis) patients [abstract]. Kidney International 1988;34(4):558. [CENTRAL: CN‐00644276]
-
- Koene R, Frenken L. Does treatment of predialysis patients with recombinant human erythropoietin compromise renal function?. Nefrologia 1990;10(Suppl 2):131‐6. - PubMed
-
- Koene RA, Frenken LA. Does treatment of predialysis patients with recombinant human erythropoietin compromise renal function?. Contributions to Nephrology 1990;87:105‐12. [MEDLINE: ] - PubMed
Gertz 2012 {published data only}
-
- Gertz B, Kes P, Essaian A, Bias P, Buchner A, Zellner D. Epoetin theta: efficacy and safety of subcutaneous administration in anemic pre‐dialysis patients in the maintenance phase in comparison to epoetin beta. Current Medical Research & Opinion 2012;28(7):1101‐10. [MEDLINE: ] - PubMed
Haag‐Weber 2012 {published data only}
-
- Haag‐Weber M, Eckardt KU, Horl WH, Roger SD, Vetter A, Roth K. Safety, immunogenicity and efficacy of subcutaneous biosimilar epoetin‐alpha (HX575) in non‐dialysis patients with renal anemia: a multi‐center, randomized, double‐blind study. Clinical Nephrology 2012;77(1):8‐17. [MEDLINE: ] - PubMed
Knebel 2008 {published data only}
-
- Knebel W, Palmen M, Dowell JA, Gastonguay M. Population pharmacokinetic modeling of epoetin delta in pediatric patients with chronic kidney disease. Journal of Clinical Pharmacology 2008;48(7):837‐48. [MEDLINE: ] - PubMed
Kronborg 1994 {published data only}
-
- Kronborg J, Willassen Y, Stromsaether CE, Paulsen D. Pain after subcutaneous injection of erythropoietin (EPO). A comparison of two different preparations [abstract]. Journal of the American Society of Nephrology 1994;5(3):460.
Mignon 2000 {published data only}
-
- Mignon F, Andrassy K, Esnault VL, Kuhlmann MK, Sinnassamy P. Epoetin delta corrects and maintains haemoglobin in pre‐end stage renal disease [abstract]. 38th Congress. European Renal Association. European Dialysis and Transplantation Association; 2001 Jun 24‐27; Vienna, Austria. 2001:140. [CENTRAL: CN‐00461317]
-
- Mignon F, Andrassy K, Esnault VL, Kuhlmann MK, Sinnassamy P, European HMR4396 Study Group. Novel human erythropoietin corrects and maintains hemoglobin in pre‐end stage renal disease (ESRD) when administered twice weekly subcutaneously [abstract no: A0384]. Journal of the American Society of Nephrology 2000;11(Sept):71A. [MEDLINE: ]
-
- Pratt RD. Epoetin delta for the treatment of anemia in patients with CKD not requiring hemodialysis [abstract no: TH‐PO377]. Journal of the American Society of Nephrology 2006;17(Abstracts):187A. [CENTRAL: CN‐00765050]
Pergola 2009 {published data only}
-
- Pergola PE, Gartenberg G, Fu M, Bowers P. Extended dosing regimens of epoetin alfa (EPO) in EPO‐naive, pre‐dialysis subjects with anemia of chronic kidney disease (CKD): an open‐label randomized study [abstract no: F‐PO1833]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):523A. [CENTRAL: CN‐00747297]
Pergola 2010 {published data only}
-
- Pergola P, Gartenberg G, Fu M, Bowers P, Sun S. Every 2 and 4 week dosing with epoetin alfa is non‐inferior to once weekly dosing in pre‐dialysis subjects with anemia of chronic kidney disease: an open‐label randomized study [abstract no: F‐PO1095]. Journal of the American Society of Nephrology 2009;20(Abstracts):364A.
-
- Pergola PE, Gartenberg G, Fu M, Sun S, Wolfson M, Bowers P. A randomized controlled study comparing once‐weekly to every‐2‐week and every‐4‐week dosing of epoetin alfa in CKD patients with anemia. Clinical Journal of the American Society of Nephrology: CJASN 2010;5(4):598‐606. [MEDLINE: ] - PMC - PubMed
PROMPT Study 2005 {published data only}
-
- Provenzano R, Bhaduri S, Singh AK, PROMPT Study Group. Extended epoetin alfa dosing as maintenance treatment for the anemia of chronic kidney disease: The PROMPT study. Clinical Nephrology 2005;64(2):113‐23. [MEDLINE: ] - PubMed
-
- Provenzano R, Bhaduri S, Tang KL, Klausner M, Singh A. Prospective, randomized evaluation of extended epoetin alfa dosing for treatment of anemia of chronic kidney disease (CKD): PROMPT interim analysis. [abstract no: SA‐PO729]. Journal of the American Society of Nephrology 2003;14(Nov):457A‐8A. [CENTRAL: CN‐00550618]
-
- Provenzano R, Bhaduri S, Tang L, Klausner M, Singh AK. Randomized, controlled trial of extended epoetin alfa dosing for the treatment of patients with the anemia of chronic kidney disease: final results from the PROMPT Study. [abstract no: SU‐PO055]. Journal of the American Society of Nephrology 2004;15(Oct):544A. [CENTRAL: CN‐00583874]
-
- Provenzano R, Singh AK. Hemoglobin maintenance with use of extended dosing of epoetin alfa in patients with diabetes and anemia of chronic kidney disease. Endocrine Practice 2007;13(3):251‐9. [MEDLINE: ] - PubMed
Sohmiya 1998 {published data only}
-
- Sohmiya M, Kakiba T, Kato Y. Therapeutic use of continuous subcutaneous infusion of recombinant human erythropoietin in malnourished predialysis anemic patients with diabetic nephropathy. European Journal of Endocrinology 1998;139(4):367‐70. [MEDLINE: ] - PubMed
Spinowitz 2008 {published data only}
-
- McGowan T, Tang L, Wolfson M. Analysis of hemoglobin decline following hemoglobin increase to >12 g/dL when initiating epoetin alfa using extended dosing regimens [abstract no: SU‐PO786]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):758A. [CENTRAL: CN‐00763607]
-
- McGowan T, Vaccaro NM, Beaver JS, Massarella J, Wolfson M. Pharmacokinetic and pharmacodynamic profiles of extended dosing of epoetin alfa in anemic patients who have chronic kidney disease and are not on dialysis. Clinical Journal of the American Society of Nephrology: CJASN 2008;3(4):1006‐14. [MEDLINE: ] - PMC - PubMed
-
- Spinowitz B, Germain M, Benz R, Wolfson M, McGowan T, Tang KL, et al. A randomized study of extended dosing regimens for initiation of epoetin alfa treatment for anemia of chronic kidney disease. Clinical Journal of the American Society of Nephrology: CJASN 2008;3(4):1015‐21. [MEDLINE: ] - PMC - PubMed
-
- Wolfson M, Spinowitz B, Germain M, Benz R, Epoetin AEDSG. Extended dosing regimens for initiation of epoetin alfa for treatment of anemia of chronic kidney disease [abstract no: 238]. American Journal of Kidney Diseases 2007;49(4):B84. [CENTRAL: CN‐00644242]
References to studies excluded from this review
Brown 1988 {published data only}
-
- Brown CD, Kieran M, Dosunmu BV, Zhao Z, Larson RH, Friedman EA. Raised hematocrit (HCT) persists six‐weeks after stopping treatment with human recombinant erythropoietin (r‐HuEPO) in azotemic anemic patients [abstract]. Kidney International 1988;33(1):184. [CENTRAL: CN‐00602117]
Clyne 1992 {published data only}
-
- Clyne N, Jogestrand T. Effect of erythropoietin treatment on physical exercise capacity and on renal function in predialytic uremic patients. Nephron 1992;60(4):390‐6. [MEDLINE: ] - PubMed
Duliege 2005 {published data only}
-
- Duliege AM, Macdougall I, Duncan N, Wessels D, Iwashita J, Schatz P, et al. Hematide, a synthetic peptide‐based erythropoiesis stimulating agent (ESA), demonstrates erythropoietic activity in a phase 2 single dose, dose escalating study in patients with chronic kidney disease (CKD) [abstract no: 3532]. Blood 2005;106(11 Pt 1):986. [CENTRAL: CN‐00624867]
-
- Duliege AM, Macdougall IC, Goldsmith D, Wessels D, Iwashita J, Schatz P, et al. HematideTM, a synthetic peptide‐based erythropoiesis stimulating agent (ESA), demonstrates erythropoietic activity in single dose studies in normal healthy volunteers (HV) and in patients with chronic kidney disease (CKD) [abstract no: SP420]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv155. [CENTRAL: CN‐00797702]
Furukawa 1992 {published data only}
-
- Furukawa A, Numata A, Imagawa A, Kaifu Y, Sumikura T, Miyake H, et al. Study of recombinant human erythropoietin treatment on the anemia of predialysis patients. Nippon Jinzo Gakkai Shi [Japanese Journal of Nephrology] 1992, (6):693‐700. [MEDLINE: ] - PubMed
Li 2004 {published data only}
-
- Li QF, Ma QY, Zhu CF. Effect of combination of bushen jianpi recipe and erythropoietin on serum tumor necrosis factor alpha in patients with anemia. Zhongguo Zhongxiyi Jiehe Zazhi [Chinese Journal of Integrated Traditional & Western Medicine] 2004;24(2):106‐8. [MEDLINE: ] - PubMed
Meloni 2003 {published data only}
-
- Meloni C, Tozzo C, Rossi V, Borzi M, Flamini M, Grotta BD, et al. Early anaemia correction with EPO: one year effects on LVH and progression of chronic renal failure (CRF) in predialysis patients (PTS) [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):157. [CENTRAL: CN‐00446732]
NCT00240734 {published data only}
-
- NCT00240734. A randomized, double‐blind, placebo‐controlled study evaluating weekly epoetin alfa (PROCRIT) administration on hemoglobin response and safety in diabetic subjects with the anemia of chronic kidney disease (CKD). www.clinicaltrials.gov/ct2/show/NCT00240734 (accessed 15 December 2016).
NCT00492427 {published data only}
-
- Baba T. Correction and maintenance study of subcutaneous injections of R744 to predialysis patients (phase III, comparative study in comparison with epoetin beta). www.clinicaltrials.gov/ct2/show/NCT00492427 (accessed 15 December 2016).
NCT00563355 {published data only}
-
- Wang AYM. A prospective randomised controlled trial to study the effects of recombinant human erythropoietin on the progression of atherosclerosis, cardiovascular function, nutrition and residual renal function in pre‐dialysis chronic renal failure patients. www.clinicaltrials.gov/ct2/show/NCT00563355 (accessed 15 December 2016).
Patel 2012 {published data only}
-
- Patel M, Thimons DG, Winston JL, Langholff W, McGowan T. An open‐label, randomized, multicenter, controlled study of epoetin alfa for the treatment of anemia of chronic kidney disease in the long term care setting. Journal of the American Medical Directors Association 2012;13(3):244‐8. [MEDLINE: ] - PubMed
Schwartz 1989 {published data only}
-
- Prior JE, Terzian A, Schwartz AB, Kim KE, Mintz GS, Kahn SB. Prolonged RBC survival and hematopoietic response to recombinant human erythropoietin (rHuEPO) in chronic renal failure (CRF) [abstract]. Kidney International 1989;35(1):318. [CENTRAL: CN‐00766056]
-
- Schwartz AB, Mintz GS, Kim KE, Prior JE, Kahn SB. Recombinant human erythropoietin (rHuEPO) increases MAP, TPRI and systolic and diastolic dysfunction with increased impedance to LV ejection due to increased HCT and RBC mass in PTS with CRF [abstract]. Kidney International 1989;35(1):334. [CENTRAL: CN‐00766227]
Shaheen 1983 {published data only}
-
- Shaheen FA, Al‐Aqeil NA, Badawi L, Sheikh IA, Shalabi NM, Adiku W, et al. Correction of anemia by erythropoietin in pre‐dialysis patients. Saudi Kidney Diseases & Transplantation Bulletin 1983;4(3):215‐9. [CENTRAL: CN‐00525736]
Singh 1999 {published data only}
-
- Singh NP, Aggarwal L, Singh T, Anuradha S, Kohli R. Anaemia, iron studies and erythropoietin in patients of chronic renal failure. Journal of the Association of Physicians of India 1999;47(3):284‐90. [MEDLINE: ] - PubMed
Teehan 1990 {published data only}
-
- Teehan BP, Benz RL, Sigler MH, Brown JM. Early intervention with recombinant human erythropoietin therapy. Seminars in Nephrology 1990;10(2 Suppl 1):28‐34. [MEDLINE: ] - PubMed
Teplan 1995 {published data only}
-
- Teplan V, Schuck O, Mengerova O, Stollova M. Recombinant human erythropoietin (r‐hu EPO) and keto amino acids (KA) in predialysis: an accelerated metabolic effect [abstract]. ISN XIII International Congress of Nephrology; 1995 Jul 2‐6; Madrid, Spain. 1995:503. [CENTRAL: CN‐00509503]
Yamazaki 1993 {published data only}
-
- Yamazaki C, Watanabe Y, Sakamoto N. Pharmacokinetic study of recombinant human erythropoietin treatment in pre‐dialysis end stage renal disease patients. Nippon Jinzo Gakkai Shi [Japanese Journal of Nephrology] 1993;35(11):1233‐42. [MEDLINE: ] - PubMed
Zheng 1992 {published data only}
-
- Zheng FL, Bi ZQ, Yang ZP, Li XW, Pu YF, Duan L. Effect of administration of low doses of rhuEPO in predialysis patients [abstract]. 9th Asian Colloquium in Nephrology; 1992 May 17‐21; Seoul, Korea. 1992:175. [CENTRAL: CN‐00462065]
References to studies awaiting assessment
NCT01576341 {published and unpublished data}
-
- NCT01576341. Open label, single arm, multicenter study to evaluate the safety and immunogenicity of HX575 epoetin alfa in the treatment of anemia associated with chronic kidney disease in pre‐dialysis and dialysis patients. www.clinicaltrials.gov/ct2/show/results/NCT01576341 (accessed 15 December 2016).
NCT01693029 {published data only}
-
- NCT01693029. Randomized, double‐blind, parallel group, multicenter study to evaluate the efficacy and safety of HX575 epoetin alfa vs. US licensed epoetin alfa (Epogen®/Procrit®) in the treatment of anemia associated with chronic kidney disease. www.clinicaltrials.gov/ct2/show/NCT01693029 (accessed 15 December 2016).
Additional references
Astor 2002
-
- Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988‐1994). Archives of Internal Medicine 2002;162(2):1401‐8. [MEDLINE: ] - PubMed
Bernhardt 2010
Big Molecule Watch Blog 2015
-
- FDA rejects Hospira's Epoetin Biosimilar. www.bigmoleculewatch.com/2015/10/27/fda‐rejects‐hospiras‐epogen‐biosimilar/ 2015.
Canadian EPO 1990
Cannella 1990
-
- Cannella G, Canna G, Sandrini M, Gaggiotti M, Nordio G, Movilli E, et al. Renormalization of high cardiac output and of left ventricular size following long‐term recombinant human erythropoietin treatment of anemic dialyzed uremic patients. Clinical Nephrology 1990;34(6):272‐8. [MEDLINE: ] - PubMed
Cody 2005
Cody 2016
Dmtrieva 2013
-
- Dmitrieva O, Lusignan S, Macdougall IC, Gallagher H, Tomson C, Harris K, et al. Association of anaemia in primary care patients with chronic kidney disease: cross sectional study of quality improvement in chronic kidney disease (QICKD) trial data. BMC Nephrology 2013;14:24. [MEDLINE: ] - PMC - PubMed
Drueke 2006
-
- Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. New England Journal of Medicine 2006;355(20):2071‐84. [MEDLINE: ] - PubMed
ERBP 2009
-
- Locatelli F, Covic A, Eckardt KU, Wiecek A, Vanholder R. ERA‐EDTA ERBP Advisory Board. Anaemia management in patients with chronic kidney disease: a position statement by the Anaemia Working Group of European Renal Best Practice (ERBP). Nephrology Dialysis Transplantation 2009;24(2):348‐54. [MEDLINE: ] - PubMed
Eschbach 1987
-
- Eschbach JW, Egrie JC, Downing MR, Browne JK, Adamson JW. Correction of the anemia of end‐stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. New England Journal of Medicine 1987;316(2):73‐8. [MEDLINE: ] - PubMed
GRADE 2008
GRADE 2011a
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE Guidelines:1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [MEDLINE: ] - PubMed
GRADE 2011b
-
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE Guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [MEDLINE: ] - PubMed
Hahn 2014
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011. Available from www.cochrane‐handbook.org.
Hsu 2002
-
- Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. Journal of the American Society of Nephrology 2002;13(2):504‐10. [MEDLINE: ] - PubMed
KDIGO 2012
-
- KDIGO. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney International ‐ Supplement 2012;2(4):279‐335.
Koch 1991
-
- Koch KM, Frei U. Treatment of renal anemia, 1960‐1990. Advances in Nephrology From the Necker Hospital 1991;20:19‐30. [MEDLINE: ] - PubMed
Lin 1985
Locatelli 2011
-
- Locatelli F, Vecchio L. Erythropoiesis‐stimulating agents in renal medicine. Oncologist 2011;16 Suppl 3:19‐24. [MEDLINE: ] - PubMed
Lundin 1989
-
- Lundin AP. Quality of life: subjective and objective improvements with recombinant human erythropoietin therapy. Seminars in Nephrology 1989;9(1 Suppl 1):22‐9. [MEDLINE: ] - PubMed
Macdougall 1999
-
- Macdougall IC, Gray SJ, Elston O, Breen C, Jenkins B, Browne J, et al. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. Journal of American Society of Nephrology 1999;10(11):2392‐5. [MEDLINE: ] - PubMed
Macdougall 2005
-
- Macdougall IC. CERA (Continuous Erythropoietin Receptor Activator): a new erythropoiesis‐stimulating agent for the treatment of anemia. Current Hematology Reports 2005;4(6):436‐40. [MEDLINE: ] - PubMed
Mikhail 2013
-
- Mikhail A, Farouk M. Epoetin biosimilars in Europe: five years on. Advances in Therapy 2013;30(1):28‐40. [MEDLINE: ] - PubMed
NICE 2015
-
- NICE Guideline. Chronic kidney disease: managing anaemia. Published: 3 June 2015. www.nice.org.uk/guidance/ng8 (accessed 15 December 2016).
Palmer 2010
-
- Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Tonelli M, Garg AX, et al. Meta analysis: erythropoiesis stimulating agents in patients with chronic kidney disease. Annals of Internal Medicine 2010;153(1):23‐33. [MEDLINE: ] - PubMed
Palmer 2012
Palmer 2014a
-
- Palmer SC, Saglimbene V, Mavridis D, Salanti G, Craig JC, Tonelli M, et al. Erythropoiesis‐stimulating agents for anaemia in adults with chronic kidney disease: a network meta‐analysis. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD010590.pub2] - DOI - PMC - PubMed
Palmer 2014b
Pappas 2008
-
- Pappas KD, Gouva CD, Katopodis KP, Nikolopoulos PM, Korantzopoulos PG, Michalis LK, et al. Correction of anemia with erythropoietin in chronic kidney disease (stage 3 or 4): effects on cardiac performance. Cardiovascular Drugs & Therapy 2008;22(1):37‐44. [MEDLINE: ] - PubMed
Ritz 2000
-
- Ritz E, Eisenhacht A. Early epoetin treatment in patients with renal insufficiency. Nephrology Dialysis Transplantation 2000;15 Suppl 3:40‐4. [MEDLINE: ] - PubMed
Roth 1994
-
- Roth D, Smith RD, Schulman G, Steinman TI, Hatch FE, Rudnick MR, et al. Effects of recombinant human erythropoietin on renal function in chronic renal failure predialysis patients. American Journal of Kidney Diseases 1994;24(5):777‐84. [MEDLINE: ] - PubMed
Schellekens 2009
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Singh 2006
-
- Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. New England Journal of Medicine 2006;355(20):2085‐98. [MEDLINE: ] - PubMed
Stone 1988
-
- Stone WJ, Graber SE, Krantz SB, Dessypris EN, O'Neil VL, Olsen NJ, et al. Treatment of the anemia of predialysis patients with recombinant human erythropoietin: a randomized, placebo‐controlled trial. American Journal of the Medical Sciences 1988;296(3):171‐9. [MEDLINE: ] - PubMed
Strippoli 2006
Szczech 2008
WHO 2011
-
- World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. World Health Organization. 2011. www.who.int/vmnis/indicators/haemoglobin/en/ (accessed 15 December 2016). [WHO/NMH/NHD/MNM/11.1]
Winearls 1986
-
- Winearls CG, Oliver DO, Pippard MJ, Reid C, Downing MR, Cotes PM. Effect of human erythropoietin derived from recombinant DNA on the anaemia of patients maintained by chronic haemodialysis. Lancet 1986;2(8157):1175‐8. [MEDLINE: ] - PubMed
Wolcott 1989
-
- Wolcott DL, Marsh JT, Rue A, Carr C, Nissenson AR. Recombinant human erythropoietin treatment may improve quality of life and cognitive function in chronic hemodialysis patients. American Journal of Kidney Diseases 1989;14(6):478‐85. [MEDLINE: ] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

