Real-time oxide evolution of copper protected by graphene and boron nitride barriers
- PMID: 28067249
- PMCID: PMC5220376
- DOI: 10.1038/srep39770
Real-time oxide evolution of copper protected by graphene and boron nitride barriers
Abstract
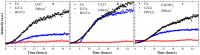
Applying protective or barrier layers to isolate a target item from the environment is a common approach to prevent or delay its degradation. The impermeability of two-dimensional materials such as graphene and hexagonal boron nitride (hBN) has generated a great deal of interest in corrosion and material science. Owing to their different electronic properties (graphene is a semimetal, whereas hBN is a wide-bandgap insulator), their protection behaviour is distinctly different. Here we investigate the performance of graphene and hBN as barrier coatings applied on copper substrates through a real-time study in two different oxidative conditions. Our findings show that the evolution of the copper oxidation is remarkably different for the two coating materials.
Figures


References
-
- Prasai D., Tuberquia J. C., Harl R. R., Jennings G. K. & Bolotin K. I. Graphene: Corrosion-inhibiting coating. ACS Nano 6, 1102–1108 (2012). - PubMed
-
- Chen S. et al. Oxidation resistance of graphene-coated Cu and Cu/Ni alloy. ACS Nano 5, 1321–1327 (2011). - PubMed
-
- Kirkland N. T., Schiller T., Medhekar N. & Birbilis N. Exploring graphene as a corrosion protection barrier. Corros. Sci. 56, 1–4 (2012).
-
- Stoot A. C., Camilli L., Spiegelhauer S.-A., Yu F. & Bøggild P. Multilayer graphene for long-term corrosion protection of stainless steel bipolar plates for polymer electrolyte membrane fuel cell. J. Power Sources 293, 846–851 (2015).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

