KANK1 inhibits cell growth by inducing apoptosis through regulating CXXC5 in human malignant peripheral nerve sheath tumors
- PMID: 28067315
- PMCID: PMC5220314
- DOI: 10.1038/srep40325
KANK1 inhibits cell growth by inducing apoptosis through regulating CXXC5 in human malignant peripheral nerve sheath tumors
Erratum in
-
Corrigendum: KANK1 inhibits cell growth by inducing apoptosis through regulating CXXC5 in human malignant peripheral nerve sheath tumors.Sci Rep. 2017 Apr 24;7:46392. doi: 10.1038/srep46392. Sci Rep. 2017. PMID: 28436992 Free PMC article. No abstract available.
Abstract
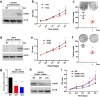
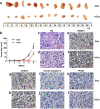
Malignant peripheral nerve sheath tumors (MPNSTs) are a type of rare sarcomas with a poor prognosis due to its highly invasive nature and limited treatment options. Currently there is no targeted-cancer therapy for this type of malignancy. Thus, it is important to identify more cancer driver genes that may serve as targets of cancer therapy. Through comparative oncogenomics, we have found that KANK1 was a candidate tumor suppressor gene (TSG) for human MPNSTs. Although KANK1 is known as a cytoskeleton regulator, its tumorigenic function in MPNSTs remains largely unknown. In this study, we report that restoration of KANK1 in human MPNST cells inhibits cell growth both in human cell culture and xenograft mice by increasing apoptosis. Consistently, knockdown of KANK1 in neurofibroma cells promoted cell growth. Using RNA-seq analysis, we identified CXXC5 and other apoptosis-related genes, and demonstrated that CXXC5 is regulated by KANK1. Knockdown of CXXC5 was found to diminish KANK1-induced apoptosis in MPNST cells. Thus, KANK1 inhibits MPNST cell growth though CXXC5 mediated apoptosis. Our results suggest that KANK1 may function as a tumor suppressor in human MPNSTs, and thus it may be useful for targeted therapy.
Figures



Similar articles
-
The Cellular Retinoic Acid Binding Protein 2 Promotes Survival of Malignant Peripheral Nerve Sheath Tumor Cells.Am J Pathol. 2017 Jul;187(7):1623-1632. doi: 10.1016/j.ajpath.2017.02.021. Epub 2017 May 11. Am J Pathol. 2017. PMID: 28502478
-
Knockdown of HMGA2 regulates the level of autophagy via interactions between MSI2 and Beclin1 to inhibit NF1-associated malignant peripheral nerve sheath tumour growth.J Exp Clin Cancer Res. 2019 May 3;38(1):185. doi: 10.1186/s13046-019-1183-2. J Exp Clin Cancer Res. 2019. PMID: 31053152 Free PMC article.
-
Oncolytic HSV and erlotinib inhibit tumor growth and angiogenesis in a novel malignant peripheral nerve sheath tumor xenograft model.Mol Ther. 2007 Feb;15(2):279-86. doi: 10.1038/sj.mt.6300038. Mol Ther. 2007. PMID: 17235305
-
Tumor suppressor mutations and growth factor signaling in the pathogenesis of NF1-associated peripheral nerve sheath tumors: II. The role of dysregulated growth factor signaling.J Neuropathol Exp Neurol. 2005 Jan;64(1):1-9. doi: 10.1093/jnen/64.1.1. J Neuropathol Exp Neurol. 2005. PMID: 15715079 Review.
-
Tumor suppressor mutations and growth factor signaling in the pathogenesis of NF1-associated peripheral nerve sheath tumors. I. The role of tumor suppressor mutations.J Neuropathol Exp Neurol. 2004 Nov;63(11):1115-23. doi: 10.1093/jnen/63.11.1115. J Neuropathol Exp Neurol. 2004. PMID: 15581179 Review.
Cited by
-
The KN Motif and Ankyrin Repeat Domains 1/CXXC Finger Protein 5 Axis Regulates Epithelial-Mesenchymal Transformation, Metastasis and Apoptosis of Gastric Cancer via Wnt Signaling.Onco Targets Ther. 2020 Jul 28;13:7343-7352. doi: 10.2147/OTT.S240991. eCollection 2020. Onco Targets Ther. 2020. PMID: 32801759 Free PMC article.
-
Multi-Omic Analysis of CIC's Functional Networks Reveals Novel Interaction Partners and a Potential Role in Mitotic Fidelity.Cancers (Basel). 2023 May 17;15(10):2805. doi: 10.3390/cancers15102805. Cancers (Basel). 2023. PMID: 37345142 Free PMC article.
-
Digital Image Analysis Applied to Tumor Cell Proliferation, Aggressiveness, and Migration-Related Protein Synthesis in Neuroblastoma 3D Models.Int J Mol Sci. 2020 Nov 17;21(22):8676. doi: 10.3390/ijms21228676. Int J Mol Sci. 2020. PMID: 33212997 Free PMC article.
-
Improving Genetic Association Studies with a Novel Methodology that Unveils the Hidden Complexity of All-Cause Heart Failure.medRxiv [Preprint]. 2023 Aug 4:2023.08.02.23293567. doi: 10.1101/2023.08.02.23293567. medRxiv. 2023. PMID: 37577697 Free PMC article. Preprint.
-
Identification of single nucleotide polymorphisms (SNPs) associated with chronic graft-versus-host disease in patients undergoing allogeneic hematopoietic cell transplantation.Support Care Cancer. 2023 Sep 21;31(10):587. doi: 10.1007/s00520-023-08044-3. Support Care Cancer. 2023. PMID: 37731134 Free PMC article.
References
-
- Ducatman B. S., Scheithauer B. W., Piepgras D. G., Reiman H. M. & Ilstrup D. M. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 57, 2006–2021 (1986). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

