The in vivo efficacy of neuraminidase inhibitors cannot be determined from the decay rates of influenza viral titers observed in treated patients
- PMID: 28067324
- PMCID: PMC5220315
- DOI: 10.1038/srep40210
The in vivo efficacy of neuraminidase inhibitors cannot be determined from the decay rates of influenza viral titers observed in treated patients
Abstract
Antiviral therapy is a first line of defence against new influenza strains. Current pandemic preparations involve stock- piling oseltamivir, an oral neuraminidase inhibitor (NAI), so rapidly determining the effectiveness of NAIs against new viral strains is vital for deciding how to use the stockpile. Previous studies have shown that it is possible to extract the drug efficacy of antivirals from the viral decay rate of chronic infections. In the present work, we use a nonlinear mathematical model representing the course of an influenza infection to explore the possibility of extracting NAI drug efficacy using only the observed viral titer decay rates seen in patients. We first show that the effect of a time-varying antiviral concentration can be accurately approximated by a constant efficacy. We derive a relationship relating the true treatment dose and time elapsed between doses to the constant drug dose required to approximate the time- varying dose. Unfortunately, even with the simplification of a constant drug efficacy, we show that the viral decay rate depends not just on drug efficacy, but also on several viral infection parameters, such as infection and production rate, so that it is not possible to extract drug efficacy from viral decay rate alone.
Conflict of interest statement
H.M.D. is currently receiving a grant from Janssen R&D, and C.A.A.B. has previously received a grant from AstraZeneca R&D.
Figures
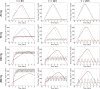
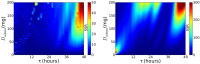
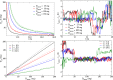
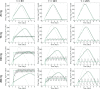

Similar articles
-
Neuraminidase inhibitors for treatment of human and avian strain influenza: A comparative modeling study.J Theor Biol. 2011 Jan 21;269(1):234-44. doi: 10.1016/j.jtbi.2010.10.017. Epub 2010 Oct 21. J Theor Biol. 2011. PMID: 20970433
-
Neuraminidase inhibitor resistance in influenza viruses.J Med Virol. 2007 Oct;79(10):1577-86. doi: 10.1002/jmv.20951. J Med Virol. 2007. PMID: 17705169 Review.
-
Neuraminidase Inhibitors in Influenza Treatment and Prevention⁻Is It Time to Call It a Day?Viruses. 2018 Aug 25;10(9):454. doi: 10.3390/v10090454. Viruses. 2018. PMID: 30149615 Free PMC article.
-
Mixed influenza A and B infections complicate the detection of influenza viruses with altered sensitivities to neuraminidase inhibitors.Antiviral Res. 2011 Jul;91(1):20-2. doi: 10.1016/j.antiviral.2011.04.010. Epub 2011 Apr 23. Antiviral Res. 2011. PMID: 21549758
-
[Research progress of neuraminidase inhibitors for anti-influenza].Yao Xue Xue Bao. 2009 Sep;44(9):935-42. Yao Xue Xue Bao. 2009. PMID: 20055166 Review. Chinese.
Cited by
-
The Mechanisms for Within-Host Influenza Virus Control Affect Model-Based Assessment and Prediction of Antiviral Treatment.Viruses. 2017 Jul 26;9(8):197. doi: 10.3390/v9080197. Viruses. 2017. PMID: 28933757 Free PMC article.
-
Quantifying the effect of remdesivir in rhesus macaques infected with SARS-CoV-2.Virology. 2020 Nov;550:61-69. doi: 10.1016/j.virol.2020.07.015. Epub 2020 Aug 23. Virology. 2020. PMID: 32882638 Free PMC article.
-
Influenza Virus Infection Model With Density Dependence Supports Biphasic Viral Decay.Front Microbiol. 2018 Jul 10;9:1554. doi: 10.3389/fmicb.2018.01554. eCollection 2018. Front Microbiol. 2018. PMID: 30042759 Free PMC article.
-
Estimation of viral kinetics model parameters in young and aged SARS-CoV-2 infected macaques.R Soc Open Sci. 2021 Nov 17;8(11):202345. doi: 10.1098/rsos.202345. eCollection 2021 Nov. R Soc Open Sci. 2021. PMID: 34804559 Free PMC article.
-
Energy Requirements for Loss of Viral Infectivity.Food Environ Virol. 2020 Dec;12(4):281-294. doi: 10.1007/s12560-020-09439-9. Epub 2020 Aug 5. Food Environ Virol. 2020. PMID: 32757142 Free PMC article.
References
-
- Centres for Disease Control and Prevention (CDC). Influenza activity – United States and worldwide, 2007–08 season. Morb Mortal Wkly Rep 57, 692–697 (2008). - PubMed
-
- Abed Y. et al.. Characterization of 2 influenza A(H3N2) clinical isolates with reduced susceptibility to neuraminidase inhibitors due to mutations in the hemagglutinin gene. J. Infect. Dis. 186, 1074–1080 (2002). - PubMed
-
- Gubareva L. V., Kaiser L. & Hayden F. G. Influenza virus neuraminidase inhibitors. Lancet 355, 827–835 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

